Abstract
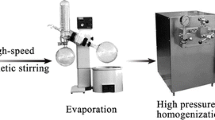
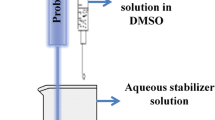
The aim of this study was to develop a nanosuspension of a highly hydrophobic drug, isradipine (ISR) by combination of anti-solvent microprecipitation and high-pressure homogenization to achieve the superior in vitro dissolution and in vivo pharmacokinetic profile. The nanosuspension was formulated using combination of stabilizers as vitamin E TPGS and sodium lauryl sulfate. The developed nanosuspension was characterized for particle size, shape, and zeta potential. The particle size of the developed ISR nanosuspension was observed to be approximately 538 nm (by laser diffraction) and 469 nm (by photon correlation spectroscopy) with −33.3 mV zeta potential. Scanning electron microscopy study revealed the good correlation with particle size measured by photon correlation spectroscopy and laser diffraction. The X-ray diffraction and differential scanning calorimetry showed that ISR was present as an amorphous state in the lyophilized form of nanosuspension. In vitro dissolution and saturation solubility study showed the dissolution rate of nanosuspensions (98.60 %) and saturation solubility (98.76 μg/ml) compared with the coarse drug (11.53 % and 14.1 μg/ml, respectively) had been significantly enhanced. The pharmacokinetic study showed that the nanosuspension exhibits increased in AUC0–48 by 2.0-fold compared to coarse suspension. Further, there was increased in C max and decreased in t max of ISR nanosuspension compared to coarse suspension of ISR. These studies proved that particle size reduction can influence ISR absorption in gastrointestinal tract and thus nanosuspension technology is responsible for enhancing oral bioavailability in rats.






Similar content being viewed by others
References
Muller RH, Peters K. Nanosuspensions for the formulation of poorly soluble drugs: I. preparation by a size reduced technique. Int J Pharm. 1998;160:229–37.
Ghosh I, Bose S, Vippagunta R, Harmon F. Nanosuspension for improving the bioavailability of a poorly soluble drug and screening of stabilizing agents to inhibit crystal growth. Int J Pharm. 2011;409:260–8.
Muller RH, Jacobs C, Kayser O. Nanosuspensions as particulate drug formulations in therapy rationale for development and what we can expect for the future. Adv Drug Deliv Rev. 2001;47:3–19.
Liversidge EM, Liversidge GG. Nanosizing for oral and parenteral drug delivery: a perspective on formulating poorly-water soluble compounds using wet media milling technology. Adv Drug Deliv Rev. 2011;63:427–40.
Rabinow BE. Nanosuspensions in drug delivery. Nat Rev Drug Discov. 2004;3:785–96.
Muller RH, Jacobs C. Buparvaquone mucoadhesive nanosuspension: preparation, optimisation and long-term stability. Int J Pharm. 2002;237(1–2):151–61.
Patravale VB, Date AA, Kulkarni RM. Nanosuspensions: a promising drug delivery strategy. J Pharma Pharmacol. 2004;56:827–40.
Singh SK, Srinivasan KK, Gowthamarajan K, Singare DS, Prakash D, Gaikwad NB. Investigation of preparation parameters of nanosuspension by top-down media milling to improve the dissolution of poorly water-soluble glyburide. Eur J Pharm Biopharm. 2011;78:441–6.
VanEerdenbrugh B, Froyen L, Martens JA, Blaton N, Augustijns P, Brewster M, et al. Characterization of physico-chemical properties and pharmaceutical performance of sucrose co-freeze-dried solid nanoparticulate powders of the anti-HIV agent loviride prepared by media milling. Int J Pharm. 2007;338:198–206.
Pua X, Suna J, Wanga Y, Wang Y, Liua X, Zhanga P, et al. Development of a chemically stable 10-hydroxycamptothecin nanosuspensions. Int J Pharm. 2009;379:167–73.
Fitton A, Benfield P. Isradipine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in cardiovascular disease. Drugs. 1990;40(1):31–74.
Walton T, Symes LR. Felodipine and isradipine: new calcium-channel-blocking agents for the treatment of hypertension. Clin Pharm. 1993;12(4):261–75.
Libo W, Jian Z, Wiwik W. Physical and chemical stability of drug nanoparticles. Adv Drug Deliv Rev. 2011;63:456–69.
Varma MVS, Panchagnula R. Enhanced oral paclitaxel absorption with vitamin E-TPGS: effect on solubility and permeability in vitro, in situ and in vivo. Eur J Pharm Sci. 2005;25:445–53.
United States Pharmacopeial Convention. United States Pharmacopeia and National Formulary (USP 30-NF 25). Rockville, MD: United States Pharmacopeial Convention. 2007; 2:531–533.
United States Pharmacopeial Convention. United States Pharmacopeia and National Formulary (USP 30-NF 25). Rockville, MD: United States Pharmacopeial Convention. 2007; 2:2432–2435.
Tse FLS, Jaffe JM, Hassell AE, Schran HF. Bioavailability of isradipine in young and old rats: effect of mode of administration. J Pharm Pharmacol. 1989;41:657–60.
Lindfors L, Forssn S, Skantze P, Skantze U, Zackrisson A, Olsson U. Amorphous drug nanosuspensions 2. Experimental determination of bulk monomer concentrations. Langmuir. 2006;22:911–6.
Muller RH, Jacobs C. Production and characterization of a budesonide nanosuspension for pulmonary administration. Pharm Res. 2002;19:189–94.
Lindfors L, Skantze P, Skantze U, Rasmusson M, Zackrisson A, Olsson U. Amorphous drug nanosuspensions. 1. Inhibition of Ostwald ripening. Langmuir. 2006;22:906–10.
Oppenheim RC, Marty JJ, Speiser PP. Injectable compositions, nanoparticles useful therein, and process of manufacturing same US 4107288. 1978.
Hintz RJ, Johnson KC. The effect of particle size distribution on dissolution rate and oral absorption. Int J Pharm. 1989;51:9–17.
Jia L, Wong H, Cerna C, Weitman SD. Effect of nanonization on absorption of 301029: ex vivo and in vivo pharmacokinetic correlations determined by liquid chromatography/mass spectrometry. Pharm Res. 2002;19:1091–6.
Acknowledgments
The authors would like to thank Kusum Healthcare, India and UGC‐SAP for providing financial assistance during the research work. The authors would like to thank AICTE/NAFETIC for providing the facility to conduct research.
Disclaimer
Authors declare that the experiments complied with current laws of India where these were performed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shelar, D.B., Pawar, S.K. & Vavia, P.R. Fabrication of isradipine nanosuspension by anti-solvent microprecipitation–high-pressure homogenization method for enhancing dissolution rate and oral bioavailability. Drug Deliv. and Transl. Res. 3, 384–391 (2013). https://doi.org/10.1007/s13346-012-0081-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-012-0081-3