Abstract
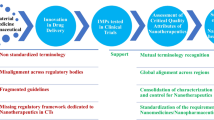
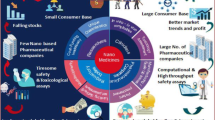
The application of the nanotechnology in medicine and pharmaceutics opens new horizons in therapeutics. Several nanomedicines are in the market and an increasing number is in clinical trials. But which is the advantage of the medicines in nanoscale? The scientists and the regulatory authorities agree that the size and consequently the physiochemical/biological properties of nanomaterials play a key role in their safety and effectiveness. Additionally, all of them agree that a new scientific-based regulatory landscape is required for the establishment of nanomedicines in the market. The aim of this review is to investigate the parameters that the scientists and the regulatory authorities should take into account in order to build up a dynamic regulatory landscape for nanomedicines. For this reason, we propose an “astrolabe-like system” as the guide for establishing the regulatory approval process. Its function is based on the different physicochemical/biological properties in comparison to low molecular weight drugs.


Similar content being viewed by others
Notes
European Medicines Agency (EMA), 2006. EMEA/CHMP/79769/2006. Reflection paper on nanotechnology-based medicinal products for human use (29 June). http://www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_ guideline/2010/01/WC500069728.pdf, Accessed date: 20 August 2018.
This “White paper” states the situation of nanomedicines and nanosimilars and suggests new scientific directions that should be considered towards a harmonized regulatory pathway.
References
Mühlebach S. Regulatory challenges of nanomedicines and their follow-on versions: a generic or similar approach? Adv Drug Deliv Rev. 2018;131:122–31. https://doi.org/10.1016/j.addr.2018.06.024.
Flühmann B, Ntai I, Borchard G, Simoens S, Mühlebach S. Nanomedicines: the magic bullets reaching their target? Eur J Pharm Sci. 2019;12:73–80. https://doi.org/10.1016/j.ejps.2018.11.019.
Crommelin DJA, Shah VP, Klebovich I, McNeil SE, Weinstein V, Flühmann B, et al. The similarity question for biologicals and non-biological complex drugs. Eur J Pharm Sci. 2015;76:10–7. https://doi.org/10.1016/j.ejps.2015.04.010.
Barenholz Y. Doxil®--the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160(2):117–34. https://doi.org/10.1016/j.jconrel.2012.03.020.
Crommelin DJ, de Vliger JS, Weinstein V, Mühlebach S, Shah VP, Schellekens H. Different pharmaceutical products need similar terminology. AAPS J. 2014;16(1):11–4. https://doi.org/10.1208/s12248-013-9532-0.
Sainz V, Conniot J, Matos AI, Reres C, Zupancic E, Moura L, et al. Regulatory aspects on nanomedicines. Biochem Biophys Res Commun. 2015;468(3):504–10. https://doi.org/10.1016/j.bbrc.2015.08.023.
Schellekens H, Stegemann S, Weinstein V, de Vlieger JS, Flühmann B, Mühlebach S, et al. How to regulate non biological complex drugs (NBCD) and their follow-on versions: points to consider. AAPS J. 2014;16(1):15–21. https://doi.org/10.1208/s12248-013-9533-z.
Mühlebach S, Borchard G, Yildiz S. Regulatory challenges and approaches to characterize nanomedicines and their follow-on similar. Nanomedicine (London). 2015;10:659–74.
Hussaarts L, Mühlebach S, Shah VP, McNeil S, Borchard G, Flühmann B, et al. Equivalence of complex drug products: advances in and challenges for current regulatory frameworks. Ann N Y Acad Sci. 2017;1407(1):39–49. https://doi.org/10.1111/nyas.13347.
Ehmann F, Sakai-Kato K, Duncan R, Hernán Pérez de la Ossa D, Pita R, Vidal JM, et al. Next-generation nanomedicines and nanosimilars: EU regulators’ initiatives relating to the development and evaluation of nanomedicines. Nanomedicine (London). 2013;8(5):849–56. https://doi.org/10.2217/nnm.13.68.
Di Francesco T, Philipp E, Borchard G. Iron sucrose: assessing the similarity between the originator drug and its intended copies. Ann N Y Acad Sci. 2017;1407(1):63–74. https://doi.org/10.1111/nyas.13517.
Hafner A, Lovrić J, Lakoš GP, Pepić I. Nanotherapeutics in EU: an overview on current state and future directions. Int J Nanomedicine. 2014;9:1005–23. https://doi.org/10.2147/IJN.S55359.
Di Francesco T, Borchard G. A robust and easily reproducible protocol for the determination of size and size distribution of iron sucrose using dynamic light scattering. J Pharm Biomed Anal. 2018;152:89–93. https://doi.org/10.1016/j.jpba.2018.01.029.
Schilt Y, Berman T, Wei X, Barenholz Y, Raviv U. Using solution X-ray scattering to determine the high-resolution structure and morphology of PEGylated liposomal doxorubicin nanodrugs. Biochim Biophys Acta. 1860;2016:108–19. https://doi.org/10.1016/j.bbagen.2015.09.012.
Wibroe PP, Ahmadvand D, Oghanian MA, Yaghmur A, Mofhimi SM. An integrated assessment of morphology, size, and complement activation of the PEGylated liposomal doxorubicin products Doxil®, Caelyx®, DOXOrubicin, and SinaDoxosome. J Control Release. 2016;221:1–8. https://doi.org/10.1016/j.jconrel.2015.11.021.
Bhattacherjee S. DLS and zeta potential-what they are and what they are not? J Control Release. 2016;235:337–51.
Soares S, Souza J, Pais A, Vitorino C. Nanomedicine: principles, properties and regulatory Issues. Front Chem. 2018;6:360. https://doi.org/10.3389/fchem.2018.00360.
Astier A, Barton Pai A, Bissig M, Crommelin DJA, Flühmann B, Hecq JD, et al. How to select a nanosimilar. Ann N Y Acad Sci. 2017;1407(1):50–62. https://doi.org/10.1111/nyas.13382.
Nicolson GL. The fluid-mosaic model of membrane structure: still relevant to understanding the structure, function and dynamics of biological membranes after more than 40years. Biochim Biophys Acta. 1838;2014:1451–66.
Shannon CE. A mathematical theory of communication. Bell Syst Tech J. 1948;327:27.
Naziris Ν, Pippa N, Pispas S, Demetzos C. The role of the information/entropy balance in self-assembly. The structural hierarchy of chimeric drug delivery nanosystems. Pharmakeftiki. 2017;29:77–82.
Tsallis C. Introduction to nonextensive statistical mechanisms. Approaching a complex world, Springer Science Business Media, LLC; 2010,
Pippa N, Dokoumetzidis A, Demetzos C, Macheras P. On the ubiquitous presence of fractals and fractal concepts in pharmaceutical sciences: a review. Int J Pharm. 2013;456(2):340–52. https://doi.org/10.1016/j.ijpharm.2013.08.087.
Pippa N, Psarommati F, Pispas S, Demetzos C. The shape/morphology balance: a study of stealth liposomes via fractal analysis and drug encapsulation. Pharm Res. 2013;30(9):2385–95. https://doi.org/10.1007/s11095-013-1082-8.
Demetzos C, Pippa N. Fractal analysis as a complementary approach to predict the stability of drug delivery nano systems in aqueous and biological media: a regulatory proposal or a dream? Int J Pharm. 2014;473:213–8. https://doi.org/10.1007/978-3-319-08927-0_27.
Demetzos C, Pippa N. Fractal geometry as a new approach for proving nanosimilarity: a reflection note. Int J Pharm. 2015;483(1–2):1–5. https://doi.org/10.1016/j.ijpharm.2015.02.008.
Demetzos C. Biophysics and thermodynamics: the scientific building blocks of bio-inspired drug delivery systems. AAPS PharmSciTech. 2015;16(3):491–5. https://doi.org/10.1208/s12249-015-0321-1.
Demetzos C. Pharmaceutical nanotechnology. Fundamentals and practical application. Springer, 2016.
Hubbell JA, Chilkoti A. Chemistry. Nanomaterials for drug delivery. Science. 2012;337:303–5.
Baars BJ, Edelman DB. Consciousness, biology and quantum hypotheses. Phys Life Rev. 2012;9:285–94.
Foffi G, Pastore A, Piazza F, Temussi PA. Macromolecular crowding: chemistry and physics meet biology. Phys Biol. 2013;10(4):040301.
Guisbiers G. Size-dependent materials properties toward a universal equation. Nanoscale Res Lett. 2010;5(7):1132–6. https://doi.org/10.1007/s11671-010-9614-1.
Mershin A, Nanopoulos DV, Skoulakis EMC. Quantum brain? Proc Acad Athens. 1999;74(A).
Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR. Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm Res. 2016;33(10):2373–87. https://doi.org/10.1007/s11095-016-1958-5.
Knoeff J, Flühmann B, Mühlebach S. Medication practice in hospitals: are nanosimilars evaluated and substituted correctly? Eur J Hosp Pharm Sci Pract. 2018;25(2):79–84. https://doi.org/10.1136/ejhpharm-2016-001059.
Rosenmayr-Templeton L. Industry update: the latest developments in therapeutic delivery. Ther Deliv. 2013;4(5):1347–52. https://doi.org/10.4155/tde.13.32.
Trikle S, McNeil SE, Mühlebach S, Bawa R, Borchard G, Barenholz YC, et al. Nanomedicines: addressing the scientific and regulatory gap. Ann N Y Acad Sci. 2014;1313:35–56. https://doi.org/10.1111/nyas.12403.
Wolfram J, Zhu M, Yang Y, Shen J, Gentile E, Paolino D, et al. Safety of nanoparticles in medicine. Curr Drug Targets. 2015;16:1671–81.
Lee VC. Progress in nanomedicine: approved and investigational nanodrugs. PT. 2017;42(12):742–55.
Marques MRC, Choo Q, Ashtikar M, Rocha TC, Bremer-Hoffmann S, Wacker MG. Nanomedicines-tiny particles and big challenges. Adv Drug Deliv Rev. 2019;In press.https://doi.org/10.1016/j.addr.2019.06.003.
Klein K, Stolk P, De Bruin ML, Leufkens HGM, Crommelin DJA, De Vlieger JSB. The EU regulatory landscape of non-biological complex drugs (NBCDs) follow-on products: observations and recommendations. Eur J Pharm Sci. 2019;133:228–35.
Rocco P, Musazzi UM, Franzè S, Minghetti P. Copies of nonbiological complex drugs: generic, hybrid or biosimilar? Drug Discov Today. 2019;24:250–5.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
“Astrolabe” was an ancient device that has been used as navigator. The Astrolabe is a complex instrument that investigates and discloses the meaning of multicomplex phenomena with precision by using its dynamic and multifunctional abilities (Scheme 1).
Rights and permissions
About this article
Cite this article
Demetzos, C., Kavatzikidou, P., Pippa, N. et al. Nanomedicines and Nanosimilars: Looking for a New and Dynamic Regulatory “Astrolabe” Inspired System. AAPS PharmSciTech 21, 65 (2020). https://doi.org/10.1208/s12249-019-1573-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1573-y