Abstract
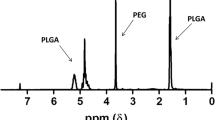
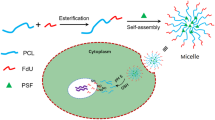
Sarsasapogenin derivative 5n (SGD 5n) is a new compound with potent antitumor efficacy, but the low solubility severely affects its absorption and bioavailability. Therefore, the SGD 5n-loaded mPEG-PLGA block copolymer micelles were developed to improve the value of SGD 5n in clinical application. The polymeric micelles were prepared by an organic solvent evaporation method, and the encapsulation efficiency (EE), drug loading (DL), critical micelle concentrations (CMC), morphology, particle size, and zeta potential were determined. The cytotoxicity was examined by the MTT assay, and the cellular uptake study was performed by confocal laser scanning microscopy. A model of tumor-bearing mouse was established to study the antitumor activity in vivo. The results demonstrated that the particle size of the prepared micelle was 124.6 ± 9.6 nm, the encapsulation efficiency was 82.0 ± 2.9%, and the drug loading was 8.3 ± 0.4%. The results of cytotoxicity and cellular uptake demonstrated that the SGD 5n-loaded micelles could efficiently enter tumor cells, and the cellular uptake of SGD 5n presented concentration and time dependence. This study demonstrated that the prepared SGD 5n-loaded polymeric micelles had significant antitumor activity and provided a basis for clinical development of new compound SGD 5n.









Similar content being viewed by others
References
Ge Y, Ma Y, Li L. The application of prodrug-based nano-drug delivery strategy in cancer combination therapy. Colloids Surf B: Biointerfaces. 2016;146:482–9. https://doi.org/10.1016/j.colsurfb.2016.06.051.
Buyel JF. Plants as sources of natural and recombinant anti-cancer agents. Biotechnol Adv. 2018;36(2):506–20. https://doi.org/10.1016/j.biotechadv.2018.02.002.
Drahl C, Cravatt BF, Sorensen EJ. Protein-reactive natural products. Angew Chem. 2005;44(36):5788–809. https://doi.org/10.1002/anie.200500900.
de Oliveira Junior RG, Christiane Adrielly AF, da Silva Almeida JRG, Grougnet R, Thiery V, Picot L. Sensitization of tumor cells to chemotherapy by natural products: a systematic review of preclinical data and molecular mechanisms. Fitoterapia. 2018;129:383–400. https://doi.org/10.1016/j.fitote.2018.02.025.
Wang W, Zhang Y, Yao G, Wang W, Shang X, Zhang Y, et al. Synthesis of new sarsasapogenin derivatives with antiproliferative and apoptotic effects in MCF-7 cells. Steroids. 2018;131:23–31. https://doi.org/10.1016/j.steroids.2018.01.001.
Lee D, Kim IY, Saha S, Choi KS. Paraptosis in the anti-cancer arsenal of natural products. Pharmacol Ther. 2016;162:120–33. https://doi.org/10.1016/j.pharmthera.2016.01.003.
Safarzadeh E, Shotorbani SS, Baradaran B. Herbal medicine as inducers of apoptosis in cancer treatment. Adv Pharm Bull. 2014;4(suppl 1):421.
Sun X-H, Zhu F-T, Zhang Y-W, Chen F-F, Yu Y, Song N-N, et al. Two new steroidal saponins isolated from Anemarrhena asphodeloides. Chin J Nat Med. 2017;15(3):220–4. https://doi.org/10.1016/s1875-5364(17)30038-9.
Shan L, Wu Y, Yuan L, Zhang Y, Xu Y, Li Y. Rapid screening of chemical constituents in Rhizoma Anemarrhenae by UPLC-Q-TOF/MS combined with data postprocessing techniques. Evid Based Complement Alternat Med. 2017;2017:4032820–14. https://doi.org/10.1155/2017/4032820.
Jiang QQ, Zhao YP, Gao WY, Li X, Huang LQ, Xiao PG. Isolation, purification, characterization and effect upon HepG2 cells of Anemaran from Rhizome Anemarrhena. Iran J Pharm Res. 2013;12(4):777–88.
Liu M, Tao L, Chau SL, Wu R, Zhang H, Yang Y, et al. Folding fan mode counter-current chromatography offers fast blind screening for drug discovery. Case study: finding anti-enterovirus 71 agents from Anemarrhena asphodeloides. J Chromatogr A. 2014;1368:116–24. https://doi.org/10.1016/j.chroma.2014.09.064.
Ji, Huang ZY, Fei CH, Xue WW, Lu TL. Comprehensive profiling and characterization of chemical constituents of rhizome of Anemarrhena asphodeloides Bge. J Chromatogr B Anal Technol Biomed Life Sci. 2017;1060:355–66. https://doi.org/10.1016/j.jchromb.2017.06.032.
Zhao Y-Z, Zhang Y-Y, Han H, Fan R-P, Hu Y, Zhong L, et al. Advances in the antitumor activities and mechanisms of action of steroidal saponins. Chin J Nat Med. 2018;16(10):732–48. https://doi.org/10.1016/s1875-5364(18)30113-4.
Wang L, Wang Z, Su S, Xing Y, Li Y, Li M, et al. Synthesis and cytotoxicity of oleanolic acid trisaccharide saponins. Carbohydr Res. 2017;442:9–16. https://doi.org/10.1016/j.carres.2017.02.010.
Yang B, Liu Z, Hu J, Lai X, Xia P. Quantitative determination of sarsasapogenin in rat plasma using liquid chromatography-tandem mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2016;1022:213–9. https://doi.org/10.1016/j.jchromb.2016.04.020.
Shen S, Zhang Y, Zhang R, Gong X. Sarsasapogenin induces apoptosis via the reactive oxygen species-mediated mitochondrial pathway and ER stress pathway in HeLa cells. Biochem Biophys Res Commun. 2013;441(2):519–24. https://doi.org/10.1016/j.bbrc.2013.10.101.
Sy LK, Lok CN, Wang JY, Liu Y, Cheng L, Wan PK, et al. Identification of “sarsasapogenin-aglyconed” timosaponins as novel Abeta-lowering modulators of amyloid precursor protein processing. Chem Sci. 2016;7(5):3206–14. https://doi.org/10.1039/c5sc02377g.
Feng B, Zhao XY, Song YZ, Liang WN, Liu JL. Sarsasapogenin reverses depressive-like behaviors and nicotinic acetylcholine receptors induced by olfactory bulbectomy. Neurosci Lett. 2017;639:173–8. https://doi.org/10.1016/j.neulet.2016.12.025.
Ni Y, Gong X-g, Lu M, Chen H-m, Wang Y. Mitochondrial ROS burst as an early sign in sarsasapogenin-induced apoptosis in HepG2 cells. Cell Biol Int. 2015;32(3):337–43.
Yin Y, Zhao XC, Wang SJ, Gao PY, Li LZ, Ikejima T, et al. Synthesis and biological evaluation of novel sarsasapogenin derivatives as potential anti-tumor agents. Steroids. 2015;93:25–31. https://doi.org/10.1016/j.steroids.2014.09.007.
Wang W, Wang D, Wang Z, Yao G, Li X, Gao P, et al. Synthesis of new sarsasapogenin derivatives with cytotoxicity and apoptosis-inducing activities in human breast cancer MCF-7 cells. Eur J Med Chem. 2017;127:62–71. https://doi.org/10.1016/j.ejmech.2016.12.011.
Sakai-Kato K, Nishiyama N, Kozaki M, Nakanishi T, Matsuda Y, Hirano M, et al. General considerations regarding the in vitro and in vivo properties of block copolymer micelle products and their evaluation. J Control Release. 2015;210:76–83. https://doi.org/10.1016/j.jconrel.2015.05.259.
Cho H, Lai TC, Tomoda K, Kwon GS. Polymeric micelles for multi-drug delivery in cancer. AAPS PharmSciTech. 2015;16(1):10–20. https://doi.org/10.1208/s12249-014-0251-3.
Liu H, Wu S, Yu J, Fan D, Ren J, Zhang L, et al. Reduction-sensitive micelles self-assembled from amphiphilic chondroitin sulfate A-deoxycholic acid conjugate for triggered release of doxorubicin. Mater Sci Eng C Mater Biol Appl. 2017;75:55–63. https://doi.org/10.1016/j.msec.2017.02.030.
Zhang J, Chen K, Ding Y, Xin X, Li W, Zhang M, et al. Self-assembly of pH-responsive dextran-g-poly(lactide-co-glycolide)-g-histidine copolymer micelles for intracellular delivery of paclitaxel and its antitumor activity. RSC Adv. 2016;6(28):23693–701.
Luong D, Kesharwani P, Deshmukh R, Mohd Amin MCI, Gupta U, Greish K, et al. PEGylated PAMAM dendrimers: enhancing efficacy and mitigating toxicity for effective anticancer drug and gene delivery. Acta Biomater. 2016;43:14–29. https://doi.org/10.1016/j.actbio.2016.07.015.
Du X, Yin S, Zhou F, Du X, Xu J, Gu X, et al. Reduction-sensitive mixed micelles for selective intracellular drug delivery to tumor cells and reversal of multidrug resistance. Int J Pharm. 2018;550(1–2):1–13. https://doi.org/10.1016/j.ijpharm.2018.08.019.
Urjita Sheth ST, Bahadur A. Preparation and characterization of anti-tubercular drugs encapsulated in polymer micelles. J Drug Delivery Sci Technol. 2018;48:422–8. https://doi.org/10.1016/j.jddst.2018.10.021.
Qiu L, Zhu M, Gong K, Peng H, Ge L, Zhao L, et al. pH-triggered degradable polymeric micelles for targeted anti-tumor drug delivery. Mater Sci Eng C Mater Biol Appl. 2017;78:912–22. https://doi.org/10.1016/j.msec.2017.04.137.
Lv S, Tang Z, Zhang D, Song W, Li M, Lin J, et al. Well-defined polymer-drug conjugate engineered with redox and pH-sensitive release mechanism for efficient delivery of paclitaxel. J Control Release. 2014;194:220–7. https://doi.org/10.1016/j.jconrel.2014.09.009.
Tang R, Ji W, Panus D, Palumbo RN, Wang C. Block copolymer micelles with acid-labile ortho ester side-chains: synthesis, characterization, and enhanced drug delivery to human glioma cells. J Control Release. 2011;151(1):18–27.
Srisang S. Layer-by-layer dip coating of Foley urinary catheters by chlorhexidine-loaded micelles. J Drug Delivery Sci Technol. 2019;49:235–42. https://doi.org/10.1016/j.jddst.2018.11.019.
Yang M, Ding H, Zhu Y, Ge Y, Li L. Co-delivery of paclitaxel and doxorubicin using mixed micelles based on the redox sensitive prodrugs. Colloids Surf B: Biointerfaces. 2019;175:126–35. https://doi.org/10.1016/j.colsurfb.2018.11.086.
Chao QXY, Wu Y, Lv Y. Matrix metalloproteinases sensitive multifunctional micelles for inhibition of metastatic tumor growth and metastasis. Powder Technol. 2018. https://doi.org/10.1016/j.powtec.2018.08.045.
Ke Z, Yang L, Wu H, Li Z, Jia X, Zhang Z. Evaluation of in vitro and in vivo antitumor effects of gambogic acid-loaded layer-by-layer self-assembled micelles. Int J Pharm. 2018;545(1–2):306–17. https://doi.org/10.1016/j.ijpharm.2018.04.016.
Hu X, Yang FF, Liu CY, Ehrhardt C, Liao YH. In vitro uptake and transport studies of PEG-PLGA polymeric micelles in respiratory epithelial cells. Eur J Pharm Biopharm. 2017;114:29–37.
Xu C, Song RJ, Lu P, Chen JC, Zhou YQ, Shen G, et al. pH-triggered charge-reversal and redox-sensitive drug-release polymer micelles codeliver doxorubicin and triptolide for prostate tumor therapy. Int J Nanomedicine. 2018;13:7229–49. https://doi.org/10.2147/ijn.s182197.
Wang Y, Luo Q, Sun R, Zha G, Li X, Shen Z, et al. Acid-triggered drug release from micelles based on amphiphilic oligo(ethylene glycol)–doxorubicin alternative copolymers. J Mater Chem B. 2014;2:7612–9. https://doi.org/10.1039/C4TB01231C.
Che-Ming Jack H, Liangfang Z. Therapeutic nanoparticles to combat cancer drug resistance. Curr Drug Metab. 2009;10(8):836–41.
Shi C, Zhang Z, Wang F, Luan Y. Active-targeting docetaxel-loaded mixed micelles for enhancing antitumor efficacy. J Mol Liq 2018;264:172–8. https://doi.org/10.1016/j.molliq.2018.05.039.
Damiani E, Solorio JA, Doyle AP, Wallace HM. How reliable are in vitro IC50 values? Values vary with cytotoxicity assays in human glioblastoma cells. Toxicol Lett. 2018;302:28–34. https://doi.org/10.1016/j.toxlet.2018.12.004.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, S., Liu, M., Wang, W. et al. Preparation and Evaluation of mPEG-PLGA Block Copolymer Micelles Loaded with a Sarsasapogenin Derivative. AAPS PharmSciTech 20, 280 (2019). https://doi.org/10.1208/s12249-019-1491-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1491-z