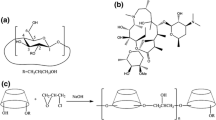
Abstract
The study mainly aimed to improve the aqueous solubility of Balofloxacin (BLFX) by preparing the inclusion complexes (ICs) of BLFX with cyclodextrins (CDs). In this study, ICs in solid state were obtained by using beta-CD (β-CD), 2-hydroxypropyl-β-CD (HP-β-CD), 2, 6-dimethyl-β-CD (DM-β-CD) through a freeze-drying technique. The formation of ICs was confirmed through Fourier-transform infrared spectroscopy, differential scanning calorimetry, powder X-ray diffraction, nuclear magnetic resonance, and scanning electron microscopy. Results demonstrated that the water solubility and dissolution rates of three ICs were distinctly improved than that of parent BLFX. Bacteriostatic experiment manifested that the antibacterial effect of BLFX was not inhibited after encapsulation in CDs. The damage of BLFX to kidney and liver cells was reduced. Consequently, successful preparation of the ICs of BLFX with CDs provided possibility for devising new dosage form of BLFX, which held great promise for further applications in clinical fields.






Similar content being viewed by others
References
Ezelarab HAA, Abbas SH, Hassan HA, Abuo-Rahma GEA. Recent updates of fluoroquinolones as antibacterial agents. Arch Pharm. 2018;351(9):e1800141. https://doi.org/10.1002/ardp.201800141.
Rizvi I, Malhotra HS, Garg RK, Kumar N, Uniyal R, Pandey S. Fluoroquinolones in the management of tuberculous meningitis: systematic review and meta-analysis. J Infect. 2018;77(4):261–75. https://doi.org/10.1016/j.jinf.2018.06.009.
Alovero F. In vitro pharmacodynamic properties of a fluoroquinolone pharmaceutical derivative: hydrochloride of ciprofloxacin–aluminium complex. Int J Antimicrob Agents. 2003;21(5):446–51. https://doi.org/10.1016/s0924-8579(03)00051-7.
DLRaCM R. Aqueous solubilities of some variously substituted quinolone antimicrobials. Int J Pharm. 1990;63:237–50.
Zhang ZH, Zhang Q, Zhang QQ, Chen C, He MY, Chen Q, et al. From a binary salt to salt co-crystals of antibacterial agent lomefloxacin with improved solubility and bioavailability. Acta Crystallogr Sect B: Struct Sci Cryst Eng Mater. 2015;71(Pt 4):437–46. https://doi.org/10.1107/S2052520615011191.
Cheng Y, Qu H, Ma M, Xu Z, Xu P, Fang Y, et al. Polyamidoamine (PAMAM) dendrimers as biocompatible carriers of quinolone antimicrobials: an in vitro study. Eur J Med Chem. 2007;42(7):1032–8. https://doi.org/10.1016/j.ejmech.2006.12.035.
Qi Y, Zhao F, Xie X, Xu X, Ma Z. Study on the cofluorescence effect of europium (III)–yttrium (III)—Balofloxacin–sodium dodecyl sulfate system and its analytical application. Spectrosc Lett. 2014;48(5):311–6. https://doi.org/10.1080/00387010.2013.879316.
Tatsuya Ito MM, Nishino T. Improved bactericidal activity of Q-35 against quinolone-resistant staphylococci. Antimicrob Agents Chemother. 1995;39:1522–5.
Osamu Kozawa TU, Matsuno H, Niwa M, Nagashima S, Kanamaru M. Comparative study of pharmacokinetics of two new fluoroquinolones, Balofloxacin and Grepafloxacin, in elderly subjects. Antimicrob Agents Chemother. 1996;40:2824–8.
Suzuki K, MH KI, Katoh S, Naide Y, Yanaoka M, Andoh S. Laboratory and clinical study of Balofloxacin (Q-35), a new fluoroquinolone, in urinary tract infection. Drugs. 1995;49:376–8.
Shelley H, Babu RJ. Role of cyclodextrins in nanoparticle-based drug delivery systems. J Pharm Sci. 2018;107(7):1741–53. https://doi.org/10.1016/j.xphs.2018.03.021.
Tang P, Sun Q, Suo Z, Zhao L, Yang H, Xiong X, et al. Rapid and efficient removal of estrogenic pollutants from water by using beta- and gamma-cyclodextrin polymers. Chem Eng J. 2018;344:514–23. https://doi.org/10.1016/j.cej.2018.03.127.
Tang P, Sun Q, Zhao L, Tang Y, Liu Y, Pu H, et al. A simple and green method to construct cyclodextrin polymer for the effective and simultaneous estrogen pollutant and metal removal. Chem Eng J. 2019;366:598–607. https://doi.org/10.1016/j.cej.2019.02.117.
Yujing Guo SG, Zhai JRY, Dong S, Wang E. Cyclodextrin functionalized graphene nanosheets with high supramolecular recognition capability synthesis and host-guest inclusion for enhanced electrochemical performance. ACS Nano. 2010;4:4001–10.
Lopedota A, Denora N, Laquintana V, Cutrignelli A, Lopalco A, Tricarico D, et al. Alginate-based hydrogel containing minoxidil/hydroxypropyl-beta-cyclodextrin inclusion complex for topical alopecia treatment. J Pharm Sci. 2018;107(4):1046–54. https://doi.org/10.1016/j.xphs.2017.11.016.
Le-Deygen IM, Skuredina AA, Uporov IV, Kudryashova EV. Thermodynamics and molecular insight in guest–host complexes of fluoroquinolones with β-cyclodextrin derivatives, as revealed by ATR-FTIR spectroscopy and molecular modeling experiments. Anal Bioanal Chem. 2017;409(27):6451–62. https://doi.org/10.1007/s00216-017-0590-5.
Shi Y, Peng J, Meng X, Huang T, Zhang J, He H. Turn-on fluorescent detection of captopril in urine samples based on hydrophilic hydroxypropyl beta-cyclodextrin polymer. Anal Bioanal Chem. 2018;410(28):7373–84. https://doi.org/10.1007/s00216-018-1343-9.
Kurkov SV, Loftsson T. Cyclodextrins. Int J Pharm. 2013;453(1):167–80. https://doi.org/10.1016/j.ijpharm.2012.06.055.
Charoenchaitrakool M, Dehghani F, Foster NR. Utilization of supercritical carbon dioxide for complex formation of ibuprofen and methyl-β-cyclodextrin. Int J Pharm. 2002;239:103–12.
Inoue Y, Watanabe S, Suzuki R, Murata I, Kanamoto I. Evaluation of actarit/γ-cyclodextrin complex prepared by different methods. J Incl Phenom Macrocycl Chem. 2014;81(1–2):161–8. https://doi.org/10.1007/s10847-014-0445-z.
Higuchi T, Connors KA. Phase solubility techniques. Adv Anal Chem Instrum 1965;4117–212.
Garnero C, Chattah AK, Aloisio C, Fabietti L, Longhi M. Improving the stability and the pharmaceutical properties of Norfloxacin form C through binary complexes with beta-cyclodextrin. AAPS PharmSciTech. 2018;19(5):2255–63. https://doi.org/10.1208/s12249-018-1033-0.
Wang L, Li S, Tang P, Yan J, Xu K, Li H. Characterization and evaluation of synthetic riluzole with beta-cyclodextrin and 2,6-di-O-methyl-beta-cyclodextrin inclusion complexes. Carbohydr Polym. 2015;129:9–16. https://doi.org/10.1016/j.carbpol.2015.04.046.
He Z, Wei G, Li N, Niu M, Gong S, Wu G, et al. CCR2 and CCR5 promote diclofenac-induced hepatotoxicity in mice. Naunyn Schmiedeberg's Arch Pharmacol. 2018;392:287–97. https://doi.org/10.1007/s00210-018-1576-3.
Yang R, Zhao Q, Hu DD, Xiao XR, Li F. Optimization of extraction and analytical protocol for mass spectrometry-based metabolomics analysis of hepatotoxicity. Biomed Chromatogr. 2018;32(12):e4359. https://doi.org/10.1002/bmc.4359.
Bian Z, Tian Y, Zhang Z, Xu F, Li J, Cao X. High performance liquid chromatography-electrospray ionization mass spectrometric determination of balofloxacin in human plasma and its pharmacokinetics. J Chromatogr B, Analytical technologies in the biomedical and life sciences. 2007;850(1–2):68–73. https://doi.org/10.1016/j.jchromb.2006.11.001.
Wang D, Chen G, Ren L. Preparation and characterization of the sulfobutylether-beta-cyclodextrin inclusion complex of amiodarone hydrochloride with enhanced oral bioavailability in fasted state. AAPS PharmSciTech. 2017;18(5):1526–35. https://doi.org/10.1208/s12249-016-0646-4.
Misiuk W, Jozefowicz M. Study on a host–guest interaction of hydroxypropyl-β-cyclodextrin with ofloxacin. J Mol Liq. 2015;202:101–6. https://doi.org/10.1016/j.molliq.2014.12.029.
Dsugi NF, Elbashir AA. Supramolecular interaction of moxifloxacin and beta-cyclodextrin spectroscopic characterization and analytical application. Spectrochim Acta A Mol Biomol Spectrosc. 2015;137:804–9. https://doi.org/10.1016/j.saa.2014.08.081.
Barbosa JS, Nolasco MM, Ribeiro-Claro P, Almeida Paz FA, Braga SS. Preformulation studies of the gamma-cyclodextrin and Montelukast inclusion compound prepared by comilling. J Pharm Sci. 2018;108:1837–47. https://doi.org/10.1016/j.xphs.2018.11.047.
Dsugi NF, Elbashir AA, Suliman FE. Supramolecular interaction of gemifloxacin and hydroxyl propyl beta-cyclodextrin spectroscopic characterization, molecular modeling and analytical application. Spectrochim Acta A Mol Biomol Spectrosc. 2015;151:360–7. https://doi.org/10.1016/j.saa.2015.06.031.
Ferreira LEN, Antunes GBM, Muniz BV, Burga-Sanchez J, de Melo NFS, Groppo FC, et al. Effects of lidocaine and the inclusion complex with 2-hydroxypropyl-beta-cyclodextrin on cell viability and proliferation of oral squamous cell carcinoma. J Pharm Pharmacol. 2018;70(7):874–82. https://doi.org/10.1111/jphp.12917.
Alves-Silva I, Sá-Barreto LCL, Lima EM, Cunha-Filho MSS. Preformulation studies of itraconazole associated with benznidazole and pharmaceutical excipients. Thermochim Acta. 2014;575:29–33. https://doi.org/10.1016/j.tca.2013.10.007.
Banchero M, Ronchetti S, Manna L. Characterization of ketoprofen/methyl-β-cyclodextrin complexes prepared using supercritical carbon dioxide. J Chem. 2013;2013:1–8. https://doi.org/10.1155/2013/583952.
Szabó Z-I, Deme R, Mucsi Z, Rusu A, Mare AD, Fiser B, et al. Equilibrium, structural and antibacterial characterization of moxifloxacin-β-cyclodextrin complex. J Mol Struct. 2018;1166:228–36. https://doi.org/10.1016/j.molstruc.2018.04.045.
Mangolim CS, Moriwaki C, Nogueira AC, Sato F, Baesso ML, Neto AM, et al. Curcumin-beta-cyclodextrin inclusion complex: stability, solubility, characterisation by FT-IR, FT-Raman, X-ray diffraction and photoacoustic spectroscopy, and food application. Food Chem. 2014;153:361–70. https://doi.org/10.1016/j.foodchem.2013.12.067.
Abdul Rauf Khan PF, Stine KJ, D’Souza VT. Methods for selective modifications of Cyclodextrins. Chem Rev. 1998;98:1977–96.
Saenger W. Cyclodextrin inclusion compounds in research and industry. Angew Chem Int Ed Engl. 1980;19:344–62.
Schneider HJ, Hacket F, Rüdiger V, Ikeda H. NMR studies of Cyclodextrins and Cyclodextrin complexes. Chem Rev. 1998;98:1755–85.
Tang P, Li S, Wang L, Yang H, Yan J, Li H. Inclusion complexes of chlorzoxazone with beta- and hydroxypropyl-beta-cyclodextrin: characterization, dissolution, and cytotoxicity. Carbohydr Polym. 2015;131:297–305. https://doi.org/10.1016/j.carbpol.2015.05.055.
Rao MRP, Chaudhari J, Trotta F, Caldera F. Investigation of Cyclodextrin-based nanosponges for solubility and bioavailability enhancement of rilpivirine. AAPS PharmSciTech. 2018;19(5):2358–69. https://doi.org/10.1208/s12249-018-1064-6.
Acknowledgments
We appreciated Hui Wang and Yueming Zhai from the Analytical & Testing Center of Sichuan University for helping with SEM and NMR characterization, respectively.
Funding
This work was supported by Sichuan Science and Technology Program (Grant No. 2018JY0188) and the Fundamental Research Funds for the Central Universities (Grant No. 2018SCU12043).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
Fig. S1 Molecular structure of β-CD, HP-β-CD, and DM-β-CD. Fig. S2 Standard curve of BLFX. Fig. S3 Phase solubility curves of BLFX with CDs. Fig. S4 Antibacterial activity result against E. coli (a) and S. aureus (b) of pure BLFX (1), BLFX/β-CD IC (2), BLFX/HP-β-CD IC (3), BLFX/DM-β-CD IC (4) (PDF 298 kb)
Rights and permissions
About this article
Cite this article
Ren, X., Qian, H., Tang, P. et al. Preparation, Characterization, and Properties of Inclusion Complexes of Balofloxacin with Cyclodextrins. AAPS PharmSciTech 20, 278 (2019). https://doi.org/10.1208/s12249-019-1425-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1425-9