Abstract
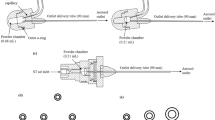
Corticosteroid resistance poses a major challenge to effective treatment of chronic obstructive pulmonary diseases. However, corticosteroid resistance can be overcome by co-administration of theophylline. The aim of this study was to formulate the corticosteroid budesonide with theophylline into inhalable dry powders intended for pulmonary combination therapy. Four types of spray-dried powders were prepared: (i) budesonide and theophylline co-dissolved and processed using a 2-fluid nozzle spray drier, (ii) budesonide nanocrystals and dissolved theophylline co-dispersed and processed using a 2-fluid nozzle spray drier, (iii) dissolved budesonide and dissolved theophylline processed using a 3-fluid nozzle spray drier, and (iv) budesonide nanocrystals and dissolved theophylline processed using a 3-fluid nozzle spray drier. Spray drying from the solutions resulted in co-amorphous (i) and partially amorphous powders (iii), whereas spray drying of the nanosuspensions resulted in crystalline products (ii and iv). Even though budesonide was amorphous in (i) and (iii), it failed to exhibit any dissolution advantage over the unprocessed budesonide. In contrast, the dissolution of budesonide from its nanocrystalline formulations, i.e., (ii) and (iv), was significantly higher compared to a physical mixture or unprocessed budesonide. Furthermore, the spray-dried powders obtained from the 2-fluid nozzle spray drier, i.e., (i) and (ii), exhibited co-deposition of budesonide and theophylline at the same weight ratio in the aerodynamic assessment using the New Generation Impactor. In contrast, the depositions of budesonide and theophylline deviated from the starting weight ratio in the aerodynamic assessment of spray-dried powders obtained from the 3-fluid nozzle spray drier, i.e., (iii) and (iv). Based on these results, the powders spray-dried from the suspension by using the 2-fluid nozzle spray drier, i.e., (ii), offered the best formulation properties given the physically stable crystalline solid-state properties and the co-deposition profile.






Similar content being viewed by others
References
Toews ML, Bylund DB. Pharmacologic principles for combination therapy. Proc Am Thorac Soc. 2005;2:282–9.
Huchon G, Magnussen H, Chuchalin A, Dymek L, Gonod FB, Bousquet J. Lung function and asthma control with beclomethasone and formoterol in a single inhaler. Respir Med. 2009;103(1):41–9.
Barnes PJ, Nicolini G, Bizzi A, Spinola M, Singh D. Do inhaled corticosteroid/long-acting beta(2)-agonist fixed combinations provide superior clinical benefits compared with separate inhalers? A literature reappraisal. Allergy Asthma Proc. 2012;33(2):140–4.
Tal A, Simon G, Vermeulen JH, Petru V, Cobos N, Everard ML, et al. Budesonide/formoterol in a single inhaler versus inhaled corticosteroids alone in the treatment of asthma. Pediatr Pulmonol. 2002;34(5):342–50.
Traini D, Adi H, Valet OK, Young PM. Preparation and evaluation of single and co-engineered combination inhalation carrier formulations for the treatment of asthma. J Pharm Sci. 2012;101(11):4267–76.
Cazzola M, Dahl R. Inhaled combination therapy with long-acting beta 2-agonists and corticosteroids in stable COPD. Chest. 2004;126(1):220–37.
Barnes PJ. Alveolar macrophages as orchestrators of COPD. Copd. 2004;1(1):59–70.
Barnes PJ. Theophylline. Am J Respir Crit Care. 2013;188(8):901–6.
Barnes PJ. New anti-inflammatory targets for chronic obstructive pulmonary disease. Nat Rev Drug Discov. 2013;12(7):543–59.
Barnes PJ. Theophylline - new perspectives for an old drug. Am J Respir Crit Care. 2003;167(6):813–8.
Parumasivam T, Chang RY, Abdelghany S, Ye TT, Britton WJ, Chan HK. Dry powder inhalable formulations for anti-tubercular therapy. Adv Drug Deliv Rev. 2016;102:83–101.
Chow AH, Tong HH, Chattopadhyay P, Shekunov BY. Particle engineering for pulmonary drug delivery. Pharm Res. 2007;24(3):411–37.
Nandiyanto ABD, Okuyama K. Progress in developing spray-drying methods for the production of controlled morphology particles: from the nanometer to submicrometer size ranges. Adv Powder Technol. 2011;22(1):1–19.
Adi H, Young PM, Chan HK, Stewart P, Agus H, Traini D. Cospray dried antibiotics for dry powder lung delivery. J Pharm Sci. 2008;97(8):3356–66.
Lee SH, Teo J, Heng D, Ng WK, Chan HK, Tan RB. Synergistic combination dry powders for inhaled antimicrobial therapy: formulation, characterization and in vitro evaluation. Eur J Pharm Biopharm. 2013;83(2):275–84.
Zhou QT, Gengenbach T, Denman JA, Yu HH, Li J, Chan HK. Synergistic antibiotic combination powders of colistin and rifampicin provide high aerosolization efficiency and moisture protection. AAPS J. 2014;16(1):37–47.
Pabari RM, Sunderland T, Ramtoola Z. Investigation of a novel 3-fluid nozzle spray drying technology for the engineering of multifunctional layered microparticles. Expert Opin Drug Deliv. 2012;9(12):1463–74.
Gharsallaoui A, Roudaut G, Chambin O, Voilley A, Saurel R. Applications of spray-drying in microencapsulation of food ingredients: an overview. Food Res Int. 2007;40(9):1107–21.
Liu T, Han M, Tian F, Cun D, Rantanen J, Yang M. Budesonide nanocrystal-loaded hyaluronic acid microparticles for inhalation: in vitro and in vivo evaluation. Carbohydr Polym. 2018;181:1143–52.
Yang JZ, Young AL, Chiang PC, Thurston A, Pretzer DK. Fluticasone and budesonide nanosuspensions for pulmonary delivery: preparation, characterization, and pharmacokinetic studies. J Pharm Sci. 2008;97(11):4869–78.
Leng D, Thanki K, Foged C, Yang M. Formulating inhalable dry powders using two-fluid and three-fluid nozzle spray drying. Pharm Res. 2018;35(12):247.
Otsuka M, Kinoshita H. Quantitative determination of hydrate content of theophylline powder by chemometric X-ray powder diffraction analysis. AAPS PharmSciTech. 2010;11(1):204–11.
Kissi EO, Kasten G, Lobmann K, Rades T, Grohganz H. The role of glass transition temperatures in coamorphous drug-amino acid formulations. Mol Pharm. 2018;submitted;15:4247–56.
Dengale SJ, Grohganz H, Rades T, Löbmann K. Recent advances in co-amorphous drug formulations. Adv Drug Deliv Rev. 2016;100:116–25.
Monnier X, Viel Q, Schamme B, Petit S, Delbreilh L, Dupray V, et al. Vitrification of two active pharmaceutical ingredients by fast scanning calorimetry: from structural relaxation to nucleation phenomena. Int J Pharm. 2018;536(1):426–33.
Blaabjerg LI, Lindenberg E, Rades T, Grohganz H, Löbmann K. Influence of preparation pathway on the glass forming ability. Int J Pharm. 2017;521(1–2):232–8.
Alzghoul A, Alhalaweh A, Mahlin D, Bergstrom CA. Experimental and computational prediction of glass transition temperature of drugs. J Chem Inf Model. 2014;54(12):3396–403.
Fox TG, Flory PJ. 2nd-order transition temperatures and related properties of polystyrene. 1. Influence of molecular weight. J Appl Phys. 1950;21(6):581–91.
Hancock BC, Zografi G. The relationship between the glass-transition temperature and the water-content of amorphous pharmaceutical solids. Pharm Res. 1994;11(4):471–7.
Trotta V, Lee WH, Loo CY, Young PM, Traini D, Scalia S. Co-spray dried resveratrol and budesonide inhalation formulation for reducing inflammation and oxidative stress in rat alveolar macrophages. Eur J Pharm Sci. 2016;86:20–8.
Nolan LM, Tajber L, McDonald BF, Barham AS, Corrigan OI, Healy AM. Excipient-free nanoporous microparticles of budesonide for pulmonary delivery. Eur J Pharm Sci. 2009;37(5):593–602.
Rattanupatam T, Srichana T. Budesonide dry powder for inhalation: effects of leucine and mannitol on the efficiency of delivery. Drug Deliv. 2014;21(6):397–405.
Muzaffar K, Kumar P. Parameter optimization for spray drying of tamarind pulp using response surface methodology. Powder Technol. 2015;279:179–84.
Pillay V, Fassihi R. Evaluation and comparison of dissolution data derived from different modified release dosage forms: an alternative method. J Control Release. 1998;55(1):45–55.
Noyes AA, Whitney WR. The rate of solution of solid substances in their own solutions. J Am Chem Soc. 1897;19:930–4.
Kanaujia P, Poovizhi P, Ng WK, Tan RBH. Amorphous formulations for dissolution and bioavailability enhancement of poorly soluble APIs. Powder Technol. 2015;285:2–15.
Perrut M, Jung J, Leboeuf F. Enhancement of dissolution rate of poorly-soluble active ingredients by supercritical fluid processes. Part I: micronization of neat particles. Int J Pharm. 2005;288(1):3–10.
Heertjes PM, Witvoet WC. Some aspects of the wetting of powders. Powder Technol. 1969;3(1):339–43.
Alonzo DE, Zhang GG, Zhou D, Gao Y, Taylor LS. Understanding the behavior of amorphous pharmaceutical systems during dissolution. Pharm Res. 2010;27(4):608–18.
Pilcer G, Rosiere R, Traina K, Sebti T, Vanderbist F, Amighi K. New co-spray-dried tobramycin nanoparticles-clarithromycin inhaled powder systems for lung infection therapy in cystic fibrosis patients. J Pharm Sci. 2013;102(6):1836–46.
Adi S, Adi H, Tang P, Traini D, Chan HK, Young PM. Micro-particle corrugation, adhesion and inhalation aerosol efficiency. Eur J Pharm Sci. 2008;35(1–2):12–8.
Acknowledgments
The study was funded by a PhD stipend of the Faculty of Health and Medical Sciences, University of Copenhagen, Denmark, and the National Natural Science Foundation of China (No. 81573380). We acknowledge the Core Facility for Integrated Microscopy, Faculty of Health and Medical Sciences, University of Copenhagen, for the morphology study of the spray-dried particles. We thank the Danish Agency for Science, the Technology and Innovation for funding the Zetasizer Nano ZS and Novo Nordisk for supporting the Next Generation Impactor. We gratefully acknowledge PhD students Junwei Wang and Yongquan Li from the University of Copenhagen for valuable scientific discussion and technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: Philip J. Kuehl and Stephen W. Stein
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 66 kb)
Rights and permissions
About this article
Cite this article
Leng, D., Kissi, E.O., Löbmann, K. et al. Design of Inhalable Solid Dosage Forms of Budesonide and Theophylline for Pulmonary Combination Therapy. AAPS PharmSciTech 20, 137 (2019). https://doi.org/10.1208/s12249-019-1344-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1344-9