Abstract
Purpose
The purpose of this study was to assess the feasibility of hydroxypropyl-β-cyclodextrin as a solubilizer for the corticosteroids prednisolone and fludrocortisone acetate in dry powder inhalation formulations.
Methods
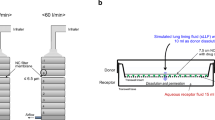
The dry particles were simultaneously produced and coated with nanosized L-leucine crystals using an aerosol flow reactor method. The aerosolization performances of carrier-free powders were studied using Easyhaler® and Twister™ at 2 and 4 kPa pressure drops over the inhalers. Drug permeation properties of the formulations were tested across a Calu-3 cell monolayer. Toxicity and reactive oxygen species induction were tested against Calu-3 and A549 cell lines.
Results
The hydroxypropyl-β-cyclodextrin in the powders promoted the dissolution of fludrocortisone the most, followed by that of prednisolone. Fine particle fractions were 52–70% from emitted doses which showed good repeatability with a coefficient variation of 0.9–0.17. In addition, hydroxypropyl-β-cyclodextrin enhanced the permeation of the corticosteroids. The powders showed no statistically significant toxicity nor reactive oxygen species induction in the tested cell lines.
Conclusions
This study demonstrated the preparation and function of fine powder formulations which combine improved dissolution of poorly soluble drugs with good aerosolization performance. These results are expected to promote particle engineering as a way to develop new types of therapeutic pulmonary powders.





Similar content being viewed by others
Abbreviations
- BLPI:
-
Berner-type low pressure impactor
- CD:
-
Cyclodextrin
- CVED :
-
The coefficient of variations of the powder emission
- ED:
-
Emitted dose
- FLU:
-
Fludrocortisone-21-acetate
- FPF:
-
Fine particle fraction
- HP-β-CD:
-
Hydroxypropyl-β-cyclodextrin
- L:
-
L-leucine
- PRE:
-
Prednisolone
- ROS:
-
Reactive oxygen species
References
Patton JS. The lungs as a portal of entry for systemic drug delivery. Proc Am Thorac Soc. 2004;1:338–44. doi:10.1513/pats.200409-049TA.
Yang W, Johnston KP, Williams III RO. Comparison of bioavailability of amorphous versus crystalline itraconazole nanoparticles via pulmonary administration in rats. Eur J Pharm Biopharm. 2010;75:33–41.
Resatz S, Gold T, Wessel M. How to choose the right solubilization technology for your API. Drug Dev Deliv. 2015;15:35–40.
Uekama K, Fujinaga T, Hirayama F, Otagiri M, Yamasaki M, Seo H, et al. Improvement of the oral bioavailability of digitalis glycosides by cyclodextrin complexation. J Pharm Sci. 1983;72:1338–41.
Otero-Espinar FJ, Anguiano-Igea S, García-Gonzalez N, Vila-Jato JL, Blanco-Méndez J. Oral bioavailability of naproxen-β-cyclodextrin inclusion compound. Int J Pharm. 1991;75:37–44.
Dhanaraju MD, Kumaran KS, Baskaran T, Moorthy MSR. Enhancement of bioavailability of griseofulvin by its complexation with β-cyclodextrin. Drug Dev Ind Pharm. 1998;24:583–7.
Malaekeh-Nikouei B, Sajadi Tabassi SA, Gerayeli G, Salmani MA, Gholamzadeh A. The effect of cyclodextrin mixtures on aqueous solubility of beclomethasone dipropionate. J Incl Phenom Macrocycl Chem. 2012;72:383–7.
Rao VM, Haslam JL, Stella VJ. Controlled and complete release of a model poorly water-soluble drug, prednisolone, from hydroxypropyl methylcellulose matrix tablets using (SBE)7m-?-cyclodextrin as a solubilizing agent. J Pharm Sci. 2001;90:807–16.
Duan MS, Zhao N, Össurardóttir ÍB, Thorsteinsson T, Loftsson T. Cyclodextrin solubilization of the antibacterial agents triclosan and triclocarban: formation of aggregates and higher-order complexes. Int J Pharm. 2005;297:213–22.
Başaran B, Bozkir A. Thermosensitive and pH induced in situ ophthalmic gelling system for ciprofloxacin hydrochloride: hydroxypropyl-beta-cyclodextrin complex. Acta Pol Pharm. 69:1137–47.
Müller BW, Brauns U. Hydroxypropyl-β cyclodextrin derivatives: influence of average degree of substitution on complexing ability and surface activity. J Pharm Sci. 1986;75:571–2.
Salem LB, Bosquillon C, Dailey LA, Delattre L, Martin GP, Evrard B, et al. Sparing methylation of β-cyclodextrin mitigates cytotoxicity and permeability induction in respiratory epithelial cell layers in vitro. J Control Release. 2009;136:110–6.
Azarbayjani AF, Lin H, Yap CW, Chan YW, Chan SY. Surface tension and wettability in transdermal delivery: a study on the in-vitro permeation of haloperidol with cyclodextrin across human epidermis. J Pharm Pharmacol. 2010;62:770–8.
Matilainen L, Toropainen T, Vihola H, Hirvonen J, Järvinen T, Jarho P, et al. In vitro toxicity and permeation of cyclodextrins in Calu-3 cells. J Control Release. 2008;126:10–6.
Ungaro F, d’Emmanuele di Villa Bianca R, Giovino C, Miro A, Sorrentino R, Quaglia F, et al. Insulin-loaded PLGA/cyclodextrin large porous particles with improved aerosolization properties: in vivo deposition and hypoglycaemic activity after delivery to rat lungs. J Control Release. 2009;135:25–34.
Kinnarinen T, Jarho P, Järvinen K, Järvinen T. Pulmonary deposition of a budesonide/gamma-cyclodextrin complex in vitro. J Control Release. 2003;90:197–205.
Cabral-Marques H, Almeida R. Optimisation of spray-drying process variables for dry powder inhalation (DPI) formulations of corticosteroid/cyclodextrin inclusion complexes. Eur J Pharm Biopharm. 2009;73:121–9.
Borgström L, Bondesson E, Morén F, Trofast E, Newman SP. Lung deposition of budesonide inhaled via Turbuhaler® : a comparison with terbutalinesulphate in normalsubjects. Eur Respir J. 1994;69–73.
Lähde A, Raula J, Kauppinen EI. Simultaneous synthesis and coating of salbutamol sulphate nanoparticles with L-leucine in the gas phase. Int J Pharm. 2008;358:256–62.
Raula J, Lähde A, Kauppinen EI. A novel gas phase method for the combined synthesis and coating of pharmaceutical particles. Pharm Res. 2008;25:242–5.
Raula J, Rahikkala A, Halkola T, Pessi J, Peltonen L, Hirvonen J, et al. Coated particle assemblies for the concomitant pulmonary administration of budesonide and salbutamol sulphate. Int J Pharm. 2013;441:248–54.
Raula J, Lähde A, Kauppinen EI. Aerosolization behavior of carrier-free L-leucine coated salbutamol sulphate powders. Int J Pharm. 2009;365:18–25.
Raula J, Kuivanen A, Lähde A, Kauppinen EI. Gas-phase synthesis of L-leucine-coated micrometer-sized salbutamol sulphate and sodium chloride particles. Powder Technol. 2008;187:289–97.
Gliński J, Chavepeyer G, Platten J-K. Surface properties of aqueous solutions of L-leucine. Biophys Chem. 2000;84:99–103.
Matubayasi N, Miyamoto H, Namihira J, Yano K, Tanaka T. Thermodynamic quantities of surface formation of aqueous electrolyte solutions. V. Aqueous solutions of aliphatic amino acids. J Colloid Interface Sci. 2002;250:431–7.
Paajanen M, Katainen J, Raula J, Kauppinen EI, Lahtinen J. Direct evidence on reduced adhesion of Salbutamol sulphate particles due to L-leucine coating. Powder Technol. 2009;192:6–11.
Eerikäinen H, Watanabe W, Kauppinen EI, Ahonen PP. Aerosol flow reactor method for synthesis of drug nanoparticles. Eur J Pharm Biopharm. 2003;55:357–60.
Zhu Y, Lee KW. Experimental study on small cyclones operating at high flowrates. J Aerosol Sci. 1999;30:1303–15.
Kauppinen E, Kurkela J, Brown D, Jokiniemi J, Mattila T. Method and apparatus for studying aerosol sources. Google Patents; 2002. Available from: http://www.google.com.au/patents/WO2002059574A1?cl=en.
Kurkela JA, Kauppinen EI, Brown DP, Jokiniemi JK, Muttonen E. A new method and apparatus for studying performance of inhalers. Respir Drug Deliv. 2002;VIII.
Hillamo RE, Kauppinen EI. On the performance of the berner low pressure impactor. Aerosol Sci Technol. 1991; 33–47.
Rahikkala A, Junnila S, Vartiainen V, Ruokolainen J, Ikkala O, Kauppinen E, et al. Polypeptide-based aerosol nanoparticles: self-assembly and control of conformation by solvent and thermal annealing. Biomacromolecules. 2014;15:2607–15.
Haghi M, Young PM, Traini D, Jaiswal R, Gong J, Bebawy M. Time- and passage-dependent characteristics of a Calu-3 respiratory epithelial cell model. Drug Dev Ind Pharm. 2010;36:1207–14.
Grainger CI, Greenwell LL, Lockley DJ, Martin GP, Forbes B. Culture of Calu-3 cells at the air interface provides a representative model of the airway epithelial barrier. Pharm Res. 2006;23:1482–90.
Bimbo LM, Mäkilä E, Laaksonen T, Lehto V-P, Salonen J, Hirvonen J, et al. Drug permeation across intestinal epithelial cells using porous silicon nanoparticles. Biomaterials. 2011;32:2625–33.
Ali HSM, York P, Blagden N, Soltanpour S, Acree WE, Jouyban A. Solubility of budesonide, hydrocortisone, and prednisolone in ethanol + water mixtures at 298.2 K. J Chem Eng Data. 2010;55:578–82.
Maynard RL. The Merck index: 12th edition 1996. Occup Environ Med. 1997;54:288.
Chew NYK, Chan H. Use of solid corrugated particles to enhance powder aerosol performance. Pharm Res. 2001;18:1570–7.
Djedaïni F, Perly B. Nuclear magnetic resonance investigation of the stoichiometries in β-cyclodextrin:steroid inclusion complexes. J Pharm Sci. 1991;80:1157–61.
Crowe A, Tan AM. Oral and inhaled corticosteroids: differences in P-glycoprotein (ABCB1) mediated efflux. Toxicol Appl Pharmacol. 2012;260:294–302.
Bimbo LM, Peltonen L, Hirvonen J, Santos HA. Toxicological profile of therapeutic nanodelivery systems. Curr Drug Metab. 2012;13:1068–86.
Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–7.
Lutter R, Van Lieshout B, Folisi C. Reduced antioxidant and cytoprotective capacity in allergy and asthma. Ann Am Thorac Soc. 2015;12:S133–6.
Nadeem A, Chhabra SK, Masood A, Raj HG. Increased oxidative stress and altered levels of antioxidants in asthma. J Allergy Clin Immunol. 2003;111:72–8.
Repine JE, Bast A, Lankhorst I. State of the art oxidative stress in chronic obstructive. Am J Respir Crit Care Med. 1997;156:341–57.
Vartiainen V, Bimbo LM, Hirvonen J, Kauppinen EI, Raula J. Drug permeation and cellular interaction of biodegradable amino acid-coated combination drug powders for pulmonary delivery. Int J Pharm. 2016;504:89–97.
ACKNOWLEDGMENTS AND DISCLOSURES
The Academy of Finland (project nos. 140362 and 276377), Orion Research Foundation, The Finnish Cultural Foundation, Biocentrum Helsinki, Finnish Medical Foundation, and the Jane and Aatos Erkko Foundation are acknowledged for financial support. The provision of facilities by Aalto University at OtaNano - Nanomicroscopy Center (Aalto-NMC) is acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Vartiainen, V., Bimbo, L.M., Hirvonen, J. et al. Aerosolization, Drug Permeation and Cellular Interaction of Dry Powder Pulmonary Formulations of Corticosteroids with Hydroxypropyl-β-Cyclodextrin as a Solubilizer. Pharm Res 34, 25–35 (2017). https://doi.org/10.1007/s11095-016-2035-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-016-2035-9



