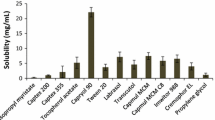
Abstract
Poor aqueous solubility and low bioavailability are limiting factors in the oral delivery of lipophilic drugs. In a formulation approach to overcome these limitations, rice bran (RB) oil was evaluated as drug carrier in the development of self-nanoemulsifying drug delivery systems (SNEDDS). The performance of RB in formulations incorporating Kolliphor RH40 or Kolliphor EL as surfactants and Transcutol HP as cosolvent was compared to a common oil vehicle, corn oil (CO). Serial dilutions of the preconcentrates were performed in various media [distilled water and simulated intestinal fluids mimicking fasted state (FaSSIF) and fed state (FeSSIF)] and at different dilution ratios to simulate the in vivo droplets’ behavior. The developed SNEDDS were assessed by means of phase separation, droplet size, polydispersity index, and ζ-potential. Complex ternary diagrams were constructed to identify compositions exhibiting monophasic behavior, droplet size < 100 nm, and polydispersity index (PDI) < 0.25. Multifactor analysis and response surface areas intended to determine the factors significantly affecting droplet size. The oil capacity to accommodate lipophilic drugs was assessed via fluorescence spectroscopy based on the solvatochromic behavior of Nile Red. Solubility studies were performed to prepare fenofibrate- and itraconazole-loaded SNEDDS and assess their droplet size, whereas dissolution experiments were conducted in simulated intestinal fluids. Caco-2 cell viability studies confirmed the safety of the SNEDDS formulations at 1:100 and 1:1000 dilutions after cell exposure in culture for 4 h. The obtained results showed similar performance between RB and CO supporting the potential of RB as oil vehicle for the effective oral delivery of lipophilic compounds.











Similar content being viewed by others
References
Cherniakov I, Domb AJ, Hoffman A. Self-nano-emulsifying drug delivery systems: an update of the biopharmaceutical aspects. Expert Opin Drug Deliv. 2015;12(7):1121–33.
Krohe M, Eek D, Mazar I, Horsfield A, Pompilus F, Friebe R, et al. Patient-reported preferences for oral versus intravenous administration for the treatment of cancer: a review of the literature. Patient Prefer Adherence. 2016;10:1609–21.
Amidon GL, Lennernäs H, Shah VP, Crison JRA. Theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12(3):413–20.
Pouton CW. Lipid formulations for oral administration of drugs: non-emulsifying, self-emulsifying and ‘self-microemulsifying’ drug delivery systems. Eur J Pharm Sci. 2000;11:S93–8.
Pouton CW. Formulation of poorly water-soluble drugs for oral administration: physicochemical and physiological issues and the lipid formulation classification system. Eur J Pharm Sci. 2006;29(3–4):278–87.
Porter CJ, Charman WN. In vitro assessment of oral lipid based formulations. Adv Drug Deliv Rev. 2001;50(Suppl 1):S127–47.
Porter CJH, Trevaskis NL, Charman WN. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat Rev Drug Discov. 2007;6(3):231–48.
Fatouros DG, Karpf DM, Nielsen FS, Mullertz A. Clinical studies with oral lipid based formulations of poorly soluble compounds. Ther Clin Risk Manag. 2007;3(4):591–604.
Kang BK, Lee JS, Chon SK, Jeong SY, Yuk SH, Khang G, et al. Development of self-microemulsifying drug delivery systems (SMEDDS) for oral bioavailability enhancement of simvastatin in beagle dogs. Int J Pharm. 2004;274(1–2):65–73.
Charman SA, Charman WN, Rogge MC, Wilson TD, Dutko FJ, Pouton CW. Self-emulsifying drug delivery systems: formulation and biopharmaceutic evaluation of an investigational lipophilic compound. Pharm Res. 1992;9(1):87–93.
Bakandritsos A, Zboril R, Bouropoulos N, Kallinteri P, Favretto ME, Parker TL, et al. The preparation of magnetically guided lipid based nanoemulsions using self-emulsifying technology. Nanotechnology. 2010;21(5):055104.
Constantinides PP. Lipid microemulsions for improving drug dissolution and oral absorption: physical and biopharmaceutical aspects. Pharm Res. 1995;12(11):1561–72.
Balakumar K, Raghavan CV, selvan NT, prasad RH, Abdu S. Self nanoemulsifying drug delivery system (SNEDDS) of rosuvastatin calcium: design, formulation, bioavailability and pharmacokinetic evaluation. Colloids Surf B Biointerfaces. 2013;112:337–43.
Qian J, Meng H, Xin L, Xia M, Shen H, Li G, et al. Self-nanoemulsifying drug delivery systems of myricetin: formulation development, characterization, and in vitro and in vivo evaluation. Colloids Surf B Biointerfaces. 2017;160:101–9.
Park JH, Kim DS, Mustapha O, Yousaf AM, Kim JS, Kim DW, et al. Comparison of a revaprazan-loaded solid dispersion, solid SNEDDS and inclusion compound: physicochemical characterisation and pharmacokinetics. Colloids Surf B Biointerfaces. 2018;162:420–6.
Khan AW, Kotta S, Ansari SH, Sharma RK, Ali J. Self-nanoemulsifying drug delivery system (SNEDDS) of the poorly water-soluble grapefruit flavonoid Naringenin: design, characterization, in vitro and in vivo evaluation. Drug Deliv. 2015;22(4):552–61.
Christiansen ML, Holm R, Abrahamsson B, Jacobsen J, Kristensen J, Andersen JR, et al. Effect of food intake and co-administration of placebo self-nanoemulsifying drug delivery systems on the absorption of cinnarizine in healthy human volunteers. Eur J Pharm Sci. 2016;84:77–82.
Fatouros DG, Deen GR, Arleth L, Bergenstahl B, Nielsen FS, Pedersen JS, et al. Structural development of self nano emulsifying drug delivery systems (SNEDDS) during in vitro lipid digestion monitored by small-angle X-ray scattering. Pharm Res. 2007;24(10):1844–53.
Fatouros D, Nielsen F, Douroumis D, Hadjileontiadis L, Mullertz A. In vitro–in vivo correlations of self-emulsifying drug delivery systems combining the dynamic lipolysis model and neuro-fuzzy networks. Eur J Pharm Biopharm. 2008;69(3):887–98.
Shahba AA-W, Mohsin K, Alanazi FK. Novel self-nanoemulsifying drug delivery systems (SNEDDS) for oral delivery of cinnarizine: design, optimization, and in-vitro assessment. AAPS PharmSciTech. 2012;13(3):967–77.
Thomas N, Holm R, Müllertz A, Rades T. In vitro and in vivo performance of novel supersaturated self-nanoemulsifying drug delivery systems (super-SNEDDS). J Control Release. 2012;160(1):25–32.
Fahmy UA, Ahmed OAA, Hosny KM. Development and evaluation of avanafil self-nanoemulsifying drug delivery system with rapid onset of action and enhanced bioavailability. AAPS PharmSciTech. 2015;16(1):53–8.
Patel J, Patel A, Raval M, Sheth N. Formulation and development of a self-nanoemulsifying drug delivery system of irbesartan. J Adv Pharm Technol Res. 2011;2(1):9–16.
Abo Enin HA, Abdel-Bar HM. Solid super saturated self-nanoemulsifying drug delivery system (sat-SNEDDS) as a promising alternative to conventional SNEDDS for improvement rosuvastatin calcium oral bioavailability. Expert Opin Drug Deliv. 2016;13(11):1513–21.
Sha X, Wu J, Chen Y, Fang X. Self-microemulsifying drug-delivery system for improved oral bioavailability of probucol: preparation and evaluation. Int J Nanomedicine. 2012;7:705–12.
Villar AMS, Naveros BC, Campmany ACC, Trenchs MA, Rocabert CB, Bellowa LH. Design and optimization of self-nanoemulsifying drug delivery systems (SNEDDS) for enhanced dissolution of gemfibrozil. Int J Pharm. 2012;431(1–2):161–75.
Nielsen FS, Petersen KB, Müllertz A. Bioavailability of probucol from lipid and surfactant based formulations in minipigs: influence of droplet size and dietary state. Eur J Pharm Biopharm. 2008;69(2):553–62.
Gershanik T. Self-dispersing lipid formulations for improving oral absorption of lipophilic drugs. Eur J Pharm Biopharm. 2000;50(1):179–88.
Brouwers J, Tack J, Lammert F, Augustijns P. Intraluminal drug and formulation behavior and integration in in vitro permeability estimation: a case study with amprenavir. J Pharm Sci. 2006;95(2):372–83.
Kataoka M, Yokoyama T, Masaoka Y, Sakuma S, Yamashita S. Estimation of P-glycoprotein-mediated efflux in the oral absorption of P-gp substrate drugs from simultaneous analysis of drug dissolution and permeation. Eur J Pharm Sci. 2011;44(4):544–51.
Irakli M, Kleisiaris F, Mygdalia A, Katsantonis D. Stabilization of rice bran and its effect on bioactive compounds content, antioxidant activity and storage stability during infrared radiation heating. J Cereal Sci. 2018;80:135–42.
Yun H-Y, Joo Lee E, Youn Chung S, Choi S-O, Kee Kim H, Kwon J-T, et al. The effects of food on the bioavailability of fenofibrate administered orally in healthy volunteers via sustained-release capsule. Clin Pharmacokinet. 2006;45(4):425–32.
Munoz A, Guichard JP, Reginault P. Micronised fenofibrate. Atherosclerosis. 1994;110:S45–8.
Najib J. Fenofibrate in the treatment of dyslipidemia: a review of the data as they relate to the new suprabioavailable tablet formulation. Clin Ther. 2002;24(12):2022–50.
Akkar A, Müller RH. Intravenous itraconazole emulsions produced by SolEmuls technology. Eur J Pharm Biopharm. 2003;56(1):29–36.
Thakkar HP, Khunt A, Dhande RD, Patel AA. Formulation and evaluation of Itraconazole nanoemulsion for enhanced oral bioavailability. J Microencapsul. 2015;32(6):559–69.
Shevchenko A, Bimbo LM, Miroshnyk I, Haarala J, Jelínková K, Syrjänen K, et al. A new cocrystal and salts of itraconazole: comparison of solid-state properties, stability and dissolution behavior. Int J Pharm. 2012;436(1–2):403–9.
Nourbehesht N, Shekarchizadeh H, Soltanizadeh N. Investigation of stability, consistency, and oil oxidation of emulsion filled gel prepared by inulin and rice bran oil using ultrasonic radiation. Ultrason Sonochem. 2018;42:585–93.
Piriyaprasarth S, Juttulapa M, Sriamornsak P. Stability of rice bran oil-in-water emulsions stabilized by pectin–zein complexes: effect of composition and order of mixing. Food Hydrocoll. 2016;61:589–98.
Srikaeo K, Pradit M. Simple techniques to increase the production yield and enhance the quality of organic rice bran oils. J Oleo Sci. 2011;60(1):1–5.
Theodoropoulos D, Rova A, Smith JR, Barbu E, Calabrese G, Vizirianakis IS, et al. Towards boron neutron capture therapy: the formulation and preliminary in vitro evaluation of liposomal vehicles for the therapeutic delivery of the dequalinium salt of bis-nido-carborane. Bioorg Med Chem Lett. 2013;23(22):6161–6.
Cendejas-Bueno E, Cuenca-Estrella M, Gomez-Lopez A. A simple, sensitive HPLC-PDA method for the quantification of itraconazole and hydroxy itraconazole in human serum: a reference laboratory experience. Diagn Microbiol Infect Dis. 2013;76(3):314–20.
Fei Y, Kostewicz ES, Sheu M-T, Dressman JB. Analysis of the enhanced oral bioavailability of fenofibrate lipid formulations in fasted humans using an in vitro–in silico–in vivo approach. Eur J Pharm Biopharm. 2013;85(3):1274–84.
Costa P, Sousa Lobo JM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13(2):123–33.
Nielsen FS, Gibault E, Ljusberg-Wahren H, Arleth L, Pedersen JS, Müllertz A. Characterization of prototype self-nanoemulsifying formulations of lipophilic compounds. J Pharm Sci. 2007;96(4):876–92.
Efiana NA, Mahmood A, Lam HT, Zupančič O, Leonaviciute G, Bernkop-Schnürch A. Improved mucoadhesive properties of self-nanoemulsifying drug delivery systems (SNEDDS) by introducing acyl chitosan. Int J Pharm. 2017;519(1–2):206–12.
Wu L, Zhang J, Watanabe W. Physical and chemical stability of drug nanoparticles. Adv Drug Deliv Rev. 2011;63(6):456–69.
Hong J-Y, Kim J-K, Song Y-K, Park J-S, Kim C-K. A new self-emulsifying formulation of itraconazole with improved dissolution and oral absorption. J Control Release. 2006;110(2):332–8.
Gupta S, Chavhan S, Sawant KK. Self-nanoemulsifying drug delivery system for adefovir dipivoxil: design, characterization, in vitro and ex vivo evaluation. Colloids Surf Physicochem Eng Asp. 2011;392(1):145–55.
Ditner C, Bravo R, Imanidis G, Kuentz M. A systematic dilution study of self-microemulsifying drug delivery systems in artificial intestinal fluid using dynamic laser light backscattering. Drug Dev Ind Pharm. 2009;35(2):199–208.
Barmpalexis P, Grypioti A, Eleftheriadis GK, Fatouros DG. Development of a new aprepitant liquisolid formulation with the aid of artificial neural networks and genetic programming. AAPS PharmSciTech. 2018;19(2):741–52.
Jores K, Haberland A, Wartewig S, Mäder K, Mehnert W. Solid lipid nanoparticles (SLN) and oil-loaded SLN studied by spectrofluorometry and Raman spectroscopy. Pharm Res. 2005;22(11):1887–97.
Hussain MD, Mohsin K, Alamari R, Ahmad A, Raish M, Alanzi F. Development of self-nanoemulsifying drug delivery systems for the enhancement of solubility and oral bioavailability of fenofibrate, a poorly water-soluble drug. Int J Nanomedicine. 2016;11:2829–38.
Strickley RG. Solubilizing excipients in oral and injectable formulations. Pharm Res. 2004;21(2):201–30.
Acknowledgements
The current work has received financial support within the project “Sustainable techno-economic solutions for the agricultural value chain” Waste-7-2015 topic H2020 690142 project (AGROCYCLE). Gattefosse (France) and BASF (Germany) are greatly acknowledged for the generously gifted formulation excipients.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editor: Sanyog Jain
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 686 kb)
Rights and permissions
About this article
Cite this article
Eleftheriadis, G.K., Mantelou, P., Karavasili, C. et al. Development and Characterization of a Self-Nanoemulsifying Drug Delivery System Comprised of Rice Bran Oil for Poorly Soluble Drugs. AAPS PharmSciTech 20, 78 (2019). https://doi.org/10.1208/s12249-018-1274-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-018-1274-y