Abstract
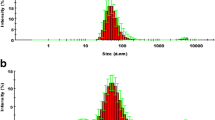
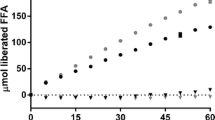
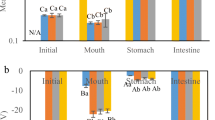
Lipid-based drug delivery systems (LbDDS), such as self-nanoemulsifying drug delivery systems (SNEDDS), constitute a prominent formulation approach for enhancing the aqueous solubility and oral bioavailability of poorly water-soluble compounds. Utilization of biorefinery wastes, such as oil from rice bran, may prove advantageous to both improving drug solubilization and absorption and to achieving sustainable agri-food waste valorization. Here, we assessed the effect of four SNEDDS compositions differing in the oil (rice bran oil and corn oil) and surfactant type (Kolliphor RH40 and EL) on the oral bioavailability of fenofibrate, a BCS class II compound. Prior to the in vivo oral administration of the SNEDDS in rats, drug solubilization was tested in vitro using the static digestion model, followed by the ex vivo permeability study of the predigested SNEDDS using the non-everted gut sac model. No significant variation was observed in the solubilization capacity within the different SNEDDS formulations. On the other hand, the ex vivo permeability data of the predigested SNEDDS correlated well with the in vivo bioavailability data designating the superiority of rice bran oil with Kolliphor EL as the surfactant, to enhance the oral absorption of fenofibrate. Results indicated that valorization of agro-industrial waste such as rice bran oil may prove useful in enhancing the oral performance of LbDDS in the case of fenofibrate, while at the same time maximizing the use of agricultural by-products via the creation of new sustainable value chains in the pharmaceutical field.






Similar content being viewed by others
References
Porter CJH, Trevaskis NL, Charman WN. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat Rev Drug Discov Engl. 2007;6:231–48.
Rehman FU, Shah KU, Shah SU, Khan IU, Khan GM, Khan A. From nanoemulsions to self-nanoemulsions, with recent advances in self-nanoemulsifying drug delivery systems (SNEDDS). Expert Opin Drug Deliv Engl. 2017;14:1325–40.
Constantinides PP. Lipid microemulsions for improving drug dissolution and oral absorption: physical and biopharmaceutical aspects. Pharm Res. 1995;12:1561–72.
Thomas N, Holm R, Rades T, Müllertz A. Characterising lipid lipolysis and its implication in lipid-based formulation development. AAPS J. 2012;14:860–71.
Norinder U, Haeberlein M. Calculated molecular properties and multivariate statistical analysis in absorption prediction. Drug Bioavailabil. 2003:358–405.
Mosgaard MD, Sassene P, Mu H, Rades T, Müllertz A. Development of a high-throughput in vitro intestinal lipolysis model for rapid screening of lipid-based drug delivery systems. Eur J Pharm Biopharm. 2015;94:493–500.
Kilic M, Dressman J. A simplified method to screen for in-vivo performance of oral lipid formulations. J Pharm Pharmacol. 2014;66:615–23.
Berthelsen R, Klitgaard M. In vitro digestion models to evaluate lipid based drug delivery systems; present status and current trends. Adv Drug Deliv Rev. 2019;142:35–49.
Keemink J, Mårtensson E, Bergström CAS. Lipolysis-permeation setup for simultaneous study of digestion and absorption in vitro. Mol Pharm. 2019;16:921–30.
Dahan A, Hoffman A. The effect of different lipid-based formulations on the oral absorption of lipophilic drugs: the ability of in vitro lipolysis and consecutive ex vivo intestinal permeability data to predict in vivo bioavailability in rats. Eur J Pharm Biopharm. 2007;67:96–105.
Bibi HA, Holm R, Bauer-Brandl A. Simultaneous lipolysis/permeation in vitro model, for the estimation of bioavailability of lipid-based drug delivery systems. Eur J Pharm Biopharm. 2017;117:300–7.
Pandey V, Kohli S. Lipids and surfactants: the inside story of lipid-based drug delivery systems. Crit Rev Ther Drug Carrier Syst. 2018;35:99–155.
Sohail M, Rakha A, Butt MS, Iqbal MJ, Rashid S. Rice bran nutraceutics: a comprehensive review. Crit Rev Food Sci Nutr. 2017;57:3771–80.
Ricciardi P, Cillari G, Carnevale Miino M, Collivignarelli MC. Valorization of agro-industry residues in the building and environmental sector: a review. Waste Manag Res J Int Solid Wastes. 2020:734242X20904426.
Pal YP, Pratap AP. Rice bran oil: a versatile source for edible and industrial applications. J Oleo Sci. 2017;66:551–6.
Ortheofer F. Rice bran oil, Bailey’s industrial oil and fat products. 6th ed. New Jersey: John Wiley & sons; 2005. p. 465–89.
Sirithunyalug B, Saenjum C, Charumanee S, Sivamaruthi BS, Chaiyasut C, Sirithunyalug J, et al. Development of colorectal-targeted dietary supplement tablets containing natural purple Rice bran oil as a colorectal chemopreventive. Nutrients. 2018;10.
Devarajan S, Singh R, Chatterjee B, Zhang B, Ali A. A blend of sesame oil and rice bran oil lowers blood pressure and improves the lipid profile in mild-to-moderate hypertensive patients. J Clin Lipidol. 2016;10:339–49.
Hunthayung K, Klinkesorn U, Hongsprabhas P, Chanput W. Controlled release and macrophage polarizing activity of cold-pressed rice bran oil in a niosome system. Food Funct. 2019;10:3272–81.
Ghatak SB, Panchal SS. Anti-diabetic activity of oryzanol and its relationship with the anti-oxidant property. Int J Diabetes Dev Ctries. 2012;32:185–92.
Wuttikul K, Boonme P. Formation of microemulsions for using as cosmeceutical delivery systems: effects of various components and characteristics of some formulations. Drug Deliv Transl Res. 2016;6:254–62.
Nourbehesht N, Shekarchizadeh H, Soltanizadeh N. Investigation of stability, consistency, and oil oxidation of emulsion filled gel prepared by inulin and rice bran oil using ultrasonic radiation. Ultrason Sonochem. 2018;42:585–93.
Granero GE, Ramachandran C, Amidon GL. Dissolution and solubility behavior of fenofibrate in sodium lauryl sulfate solutions. Drug Dev Ind Pharm. 2005;31:917–22.
Persson LC, Porter CJH, Charman WN, Bergström CAS. Computational prediction of drug solubility in lipid-based formulation excipients. Pharm Res. 2013;30:3225–37.
Pestieau A, Lebrun S, Cahay B, Brouwers A, Streel B, Cardot J-M, et al. Evaluation of different in vitro dissolution tests based on level A in vitro–in vivo correlations for fenofibrate self-emulsifying lipid-based formulations. Eur J Pharm Biopharm. 2017;112:18–29.
Tran T, Siqueira SDVS, Amenitsch H, Müllertz A, Rades T. In vitro and in vivo performance of monoacyl phospholipid-based self-emulsifying drug delivery systems. J Control Release. 2017;255:45–53.
Pestieau A, Krier F, Brouwers A, Streel B, Evrard B. Selection of a discriminant and biorelevant in vitro dissolution test for the development of fenofibrate self-emulsifying lipid-based formulations. Eur J Pharm Sci. 2016;92:212–9.
Shazly G, Mohsin K. Dissolution improvement of solid self-emulsifying drug delivery systems of fenofibrate using an inorganic high surface adsorption material. Acta Pharma. 2015;65:29–42.
Quan G, Wu Q, Zhang X, Zhan Z, Zhou C, Chen B, et al. Enhancing in vitro dissolution and in vivo bioavailability of fenofibrate by solid self-emulsifying matrix combined with SBA-15 mesoporous silica. Colloids Surf B Biointerfaces. 2016;141:476–82.
Kanaujia P, Ng WK, Tan RBH. Solid self-emulsifying drug delivery system (S-SEDDS) for improved dissolution rate of fenofibrate. J Microencapsul. 2014;31:293–8.
Shah AV, Serajuddin ATM. Development of solid self-emulsifying drug delivery system (SEDDS) I: use of poloxamer 188 as both solidifying and emulsifying agent for lipids. Pharm Res. 2012;29:2817–32.
Lee DW, Marasini N, Poudel BK, Kim JH, Cho HJ, Moon BK, et al. Application of box-Behnken design in the preparation and optimization of fenofibrate-loaded self-microemulsifying drug delivery system (SMEDDS). J Microencapsul. 2014;31:31–40.
Sunazuka Y, Ueda K, Higashi K, Tanaka Y, Moribe K. Combined effects of the drug distribution and mucus diffusion properties of self-microemulsifying drug delivery systems on the oral absorption of fenofibrate. Int J Pharm. 2018;546:263–71.
Ren S, Mu H, Alchaer F, Chtatou A, Müllertz A. Optimization of self nanoemulsifying drug delivery system for poorly water-soluble drug using response surface methodology. Drug Dev Ind Pharm. 2013;39:799–806.
Mohsin K, Alamri R, Ahmad A, Raish M, Alanazi FK, Hussain MD. Development of self-nanoemulsifying drug delivery systems for the enhancement of solubility and oral bioavailability of fenofibrate, a poorly water-soluble drug. Int J Nanomedicine. 2016;11:2829–38.
Alshamsan A, Kazi M, Badran MM, Alanazi FK. Role of alternative lipid excipients in the design of self-nanoemulsifying formulations for fenofibrate: characterization, in vitro dispersion, digestion and ex vivo gut permeation studies. Front Pharmacol. 2018;9:1219.
Tran T, Bønløkke P, Rodríguez-Rodríguez C, Nosrati Z, Esquinas PL, Borkar N, et al. Using in vitro lipolysis and SPECT/CT in vivo imaging to understand oral absorption of fenofibrate from lipid-based drug delivery systems. J Control Release. 2020;317:375–84.
Thomas N, Richter K, Pedersen TB, Holm R, Müllertz A, Rades T. In vitro lipolysis data does not adequately predict the in vivo performance of lipid-based drug delivery systems containing fenofibrate. AAPS J. 2014;16:539–49.
Eleftheriadis GK, Mantelou P, Karavasili C, Chatzopoulou P, Katsantonis D, Irakli M, et al. Development and characterization of a self-nanoemulsifying drug delivery system comprised of rice bran oil for poorly soluble drugs. AAPS PharmSciTech. 2019;20:78.
Pouton CW. Formulation of poorly water-soluble drugs for oral administration: physicochemical and physiological issues and the lipid formulation classification system. Eur J Pharm Sci. 2006;29:278–87.
Shafiq S, Shakeel F, Talegaonkar S, Ahmad FJ, Khar RK, Ali M. Development and bioavailability assessment of ramipril nanoemulsion formulation. Eur J Pharm Biopharm. 2007;66:227–43.
Kontogiannidou E, Karavasili C, Kouskoura MG, Filippousi M, Van Tendeloo G, Andreadis II, et al. In vitro and ex vivo assessment of microporous faujasite zeolite (NaX-FAU) as a carrier for the oral delivery of danazol. J Drug Deliv Sci Technol. 2019;51:177–84.
Zhang Y, Huo M, Zhou J, Xie S. PKSolver: an add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput Methods Prog Biomed. 2010;99:306–14.
Berthelsen R, Holm R, Jacobsen J, Kristensen J, Abrahamsson B, Müllertz A. Kolliphor surfactants affect solubilization and bioavailability of fenofibrate. Studies of in vitro digestion and absorption in rats. Mol Pharm. 2015;12:1062–71.
Hussain A, Singh SK, Singh N, Prasad Verma PR. In vitro–in vivo–in silico simulation studies of anti-tubercular drugs doped with a self-nanoemulsifying drug delivery system. RSC Adv. 2016;6:93147–61.
Nidhi B, Baskaran V. Influence of vegetable oils on micellization of lutein in a simulated digestion model. J Am Oil Chem Soc. 2011;88:367–72.
Acknowledgments
Gattefosse (St. Priest, France) and BASF (Ludwigshafen, Germany) are greatly acknowledged for the donation of the formulation excipients. The authors thank Dr. Maria Kollia of the Laboratory of Electron Microscopy and Microanalysis, School of Natural Sciences, University of Patras, Greece, and Prof. Nikolaos Bouropoulos from the Department of Materials Science, University of Patras, Greece, for the TEM images.
Funding
The current work has been supported by the “Sustainable techno-economic solutions for the agricultural value chain” Waste-7-2015 topic H2020 690142 project (AGROCYCLE).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karavasili, C., Andreadis, I.I., Tsantarliotou, M.P. et al. Self-Nanoemulsifying Drug Delivery Systems (SNEDDS) Containing Rice Bran Oil for Enhanced Fenofibrate Oral Delivery: In Vitro Digestion, Ex Vivo Permeability, and In Vivo Bioavailability Studies. AAPS PharmSciTech 21, 208 (2020). https://doi.org/10.1208/s12249-020-01765-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-020-01765-2