Abstract
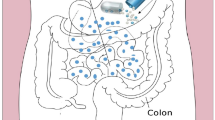
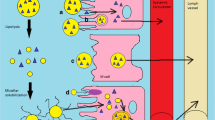
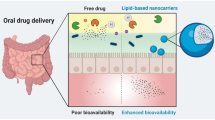
Partition coefficient (log P) is a key physicochemical characteristic of lipophilic drugs which plays a significant role in formulation development for oral administration. Lipid-based formulation strategies can increase lymphatic transport of these drugs and can enhance bioavailability many folds. The number of lipophilic drugs in pharmacopoeias and under discovery are continuously increasing and making the job of the formulation scientist difficult to develop suitable formulation of these drugs due to potent nature and water insolubility of these drugs. Recently, many natural and synthetic lipids are appearing in the market which are helpful in the development of lipid-based formulations of these types of drugs having enhanced solubility and bioavailability. One such reason for this enhanced bioavailability is the accessibility of the lymphatic transport as well as avoidance of first-pass effect. This review discusses the impact of lipophilicity in enhancing the intestinal lymphatic drug transport thereby reducing first-pass metabolism. The most appropriate strategy for developing a lipid-based formulation depending upon the degree of lipophilicity has been critically discussed and provides information on how to develop optimum formulation. Various formulation strategies are discussed in-depth by classifying lipid-based oral drug delivery systems with case studies of few marketed formulations with challenges and opportunities for the future of the formulations.





Similar content being viewed by others
References
Prabhu S, Ortega M, Ma C. Novel lipid-based formulations enhancing the in vitro dissolution and permeability characteristics of a poorly water-soluble model drug, piroxicam. Int J Pharm. 2005;301(1–2):209–16.
Lipinski CA. Drug-like properties and the causes of poor solubility and poor permeability. J Pharmacol Toxicol Methods. 2000;44(1):235–49.
Porter CJ, Charman WN. In vitro assessment of oral lipid based formulations. Adv Drug Deliv Rev. 2001;50:S127–S47.
Tiwle R, Giri TK, Tripathi DK, Jain V, Alexander A. An exhaustive review on solubility enhancement for hydrophobic compounds by possible applications of novel techniques. Trends Appl Sci Res. 2012;7(8):596.
Chaudhary A, Nagaich U, Gulati N, Sharma V, Khosa R, Partapur M. Enhancement of solubilization and bioavailability of poorly soluble drugs by physical and chemical modifications: a recent review. J Adv Pharm Educ Res. 2012;2(1):32–67.
Pouton CW. Formulation of poorly water-soluble drugs for oral administration: physicochemical and physiological issues and the lipid formulation classification system. Eur J Pharm Sci. 2006;29(3–4):278–87.
Jannin V, Musakhanian J, Marchaud D. Approaches for the development of solid and semi-solid lipid-based formulations. Adv Drug Deliv Rev. 2008;60(6):734–46.
Charman WN, Porter CJ, Mithani S, Dressman JB. Physicochemical and physiological mechanisms for the effects of food on drug absorption: the role of lipids and pH. J Pharm Sci. 1997;86(3):269–82.
Fleisher D, Li C, Zhou Y, Pao L-H, Karim A. Drug, meal and formulation interactions influencing drug absorption after oral administration. Clin Pharmacokinet. 1999;36(3):233–54.
Sahbaz Y, Williams HD, Nguyen T-H, Saunders J, Ford L, Charman SA, et al. Transformation of poorly water-soluble drugs into lipophilic ionic liquids enhances oral drug exposure from lipid based formulations. Mol Pharm. 2015;12(6):1980–91.
Yin N, Brimble M, Harris P, Wen J. Enhancing the oral bioavailability of peptide drugs by using chemical modification and other approaches. Med Chem. 2014;4:763–9.
Patil S, Vhora I, Amrutiya J, Lalani R, Misra A. Role of nanotechnology in delivery of protein and peptide drugs. Curr Pharm Des. 2015;21(29):4155–73.
New RR, Kirby CJ. Solubilisation of hydrophilic drugs in oily formulations. Adv Drug Deliv Rev. 1997;25(1):59–69.
Shiau Y-F. In: Johnson LR, editor. Lipid digestion and absorption. 2nd ed. New York: Raven; 1986. p. 1527–56.
Wang C-S. Hydrolysis of dietary glycerides and phosphoglycerides: fatty acid and positional specificity of lipases and phospholipases. Fat absorption: CRC Press; 2018. p. 83–118.
Hunt J, Knox M. A relation between the chain length of fatty acids and the slowing of gastric emptying. J Physiol. 1968;194(2):327–36.
Gibson L. Lipid-based excipients for oral drug delivery. Drugs and the Pharmaceutical Sciences. 2007;170:33.
Pouton CW. Lipid formulations for oral administration of drugs: non-emulsifying, self-emulsifying and ‘self-microemulsifying’ drug delivery systems. Eur J Pharm Sci. 2000;11:S93–S8.
Hauss DJ, Fogal SE, Ficorilli JV, Price CA, Roy T, Jayaraj AA, et al. Lipid-based delivery systems for improving the bioavailability and lymphatic transport of a poorly water-soluble LTB4 inhibitor. J Pharm Sci. 1998;87(2):164–9.
Pouton CW, Porter CJ. Formulation of lipid-based delivery systems for oral administration: materials, methods and strategies. Adv Drug Deliv Rev. 2008;60(6):625–37.
Charman W, Stella V. Estimating the maximal potential for intestinal lymphatic transport of lipophilic drug molecules. Int J Pharm. 1986;34(1–2):175–8.
Erlanson-Albertsson C. Pancreatic colipase. Structural and physiological aspects. Biochim Biophys Acta. 1992;1125(1):1–7.
Bosch H, Postema N. Haas GHd, Van Deenen L. On the positional specificity of phospholipase A from pancreas. Biochim Biophys Acta. 1965;98(3):657–9.
Hoffman N. The relationship between uptake in vitro of oleic acid and micellar solubilization. Biochim Biophys Acta Biomembr. 1970;196(2):193–203.
Westergaard H, Dietschy JM. The mechanism whereby bile acid micelles increase the rate of fatty acid and cholesterol uptake into the intestinal mucosal cell. J Clin Invest. 1976;58(1):97–108.
Simmonds W. The role of micellar solubilization in lipid absorption. Aust J Exp Biol Med Sci. 1972;50(4):403–21.
Wasan KM. Formulation and physiological and biopharmaceutical issues in the development of oral lipid-based drug delivery systems. Drug Dev Ind Pharm. 2001;27(4):267–76.
Kalepu S, Manthina M, Padavala V. Oral lipid-based drug delivery systems—an overview. Acta Pharm Sin B. 2013;3(6):361–72.
Pouton CW, Charman WN. The potential of oily formulations for drug delivery to the gastro-intestinal tract. Elsevier; 1997.
Ananthakrishnan P, Mariani G, Moresco L, Giuliano AE. The anatomy and physiology of lymphatic circulation. Radioguided Surgery: Springer; 2008. p. 57–71.
Hiroshi Y, Shozo M, Chiharu K, Hitoshi S. Bifunctional delivery system for selective transfer of bleomycin into lymphatics via enteral route. Int J Pharm. 1981;8(4):291–302.
Beier R, Gebert A. Kinetics of particle uptake in the domes of Peyer’s patches. Am J Physiol Gastrointest Liver Physiol. 1998;275(1):G130–G7.
Eldridge JH, Hammond CJ, Meulbroek JA, Staas JK, Gilley RM, Tice TR. Controlled vaccine release in the gut-associated lymphoid tissues. I. Orally administered biodegradable microspheres target the Peyer’s patches. J Control Release. 1990;11(1–3):205–14.
Ahn H, Park J-H. Liposomal delivery systems for intestinal lymphatic drug transport. Biomater Res. 2016;20(1):36.
Iqbal J, Hussain MM. Intestinal lipid absorption. Am J Physiol Endocrinol Metab. 2009;296(6):E1183–E94.
Wilson FA, Dietschy JM. The intestinal unstirred layer: its surface area and effect on active transport kinetics. Biochim Biophys Acta Biomembr. 1974;363(1):112–26.
Thomson A, Schoeller C, Keelan M, Smith L, Clandinin M. Lipid absorptions passing through the unstirred layers, brush-border membrane, and beyond. Can J Physiol Pharmacol. 1993;71(8):531–55.
Humberstone AJ, Charman WN. Lipid-based vehicles for the oral delivery of poorly water soluble drugs. Adv Drug Deliv Rev. 1997;25(1):103–28.
Yáñez JA, Wang SW, Knemeyer IW, Wirth MA, Alton KB. Intestinal lymphatic transport for drug delivery. Adv Drug Deliv Rev. 2011;63(10–11):923–42.
Wacher VJ, Silverman JA, Zhang Y, Benet LZ. Role of P-glycoprotein and cytochrome P450 3A in limiting oral absorption of peptides and peptidomimetics. J Pharm Sci. 1998;87(11):1322–30.
Borst P, Schinkel A, Smit J, Wagenaar E, Van Deemter L, Smith A, et al. Classical and novel forms of multidrug resistance and the physiological functions of P-glycoproteins in mammals. Pharmacol Ther. 1993;60(2):289–99.
Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci. 1987;84(21):7735–8.
Parkinson A. An overview of current cytochrome P450 technology for assessing the safety and efficacy of new materials. Toxicol Pathol. 1996;24(1):45–57.
Wacher VJ, Salphati L, Benet LZ. Active secretion and enterocytic drug metabolism barriers to drug absorption. Adv Drug Deliv Rev. 1996;20(1):99–112.
Zordan-Nudo T, Ling V, Liu Z, Georges E. Effects of nonionic detergents on P-glycoprotein drug binding and reversal of multidrug resistance. Cancer Res. 1993;53(24):5994–6000.
Batrakova EV, Li S, Miller DW, Kabanov AV. Pluronic P85 increases permeability of a broad spectrum of drugs in polarized BBMEC and Caco-2 cell monolayers. Pharm Res. 1999;16(9):1366–72.
Regev R, Assaraf YG, Eytan GD. Membrane fluidization by ether, other anesthetics, and certain agents abolishes P-glycoprotein ATPase activity and modulates efflux from multidrug-resistant cells. FEBS J. 1999;259(1–2):18–24.
Yoo J-SH, Smith TJ, Ning SM, Mao-Jung L, Thomas PE, Yang CS. Modulation of the levels of cytochromes P450 in rat liver and lung by dietary lipid. Biochem Pharmacol. 1992;43(12):2535–42.
Soldner A, Christians U, Susanto M, Wacher VJ, Silverman JA, Benet LZ. Grapefruit juice activates P-glycoprotein-mediated drug transport. Pharm Res. 1999;16(4):478–85.
Dahan A, Hoffman A. Rationalizing the selection of oral lipid based drug delivery systems by an in vitro dynamic lipolysis model for improved oral bioavailability of poorly water soluble drugs. J Control Release. 2008;129(1):1–10.
Savla R, Browne J, Plassat V, Wasan KM, Wasan EK. Review and analysis of FDA approved drugs using lipid-based formulations. Drug Dev Ind Pharm. 2017;43(11):1743–58.
Feeney OM, Crum MF, McEvoy CL, Trevaskis NL, Williams HD, Pouton CW, et al. 50 years of oral lipid-based formulations: provenance, progress and future perspectives. Adv Drug Deliv Rev. 2016;101:167–94.
Strickley RG. Currently marketed oral lipid-based dosage forms: drug products and excipients. Drugs and the Pharmaceutical Sciences. 2007;170:1.
Strickley RG. Solubilizing excipients in oral and injectable formulations. Pharm Res. 2004;21(2):201–30.
Alexander A. A review on novel therapeutic strategies for the enhancement of solubility for hydrophobic drugs through lipid and surfactant based self micro emulsifying drug delivery system: a novel approach. Am J Drug Disc Dev. 2012;2(4):143–83.
Charman WN, Porter CJ. Oral lipid-based formulations: using preclinical data to dictate formulation strategies for poorly water-soluble drugs. Oral lipid-based formulations: CRC Press; 2007. p. 207–28.
Griffin B. Advances in lipid-based formulations: overcoming the challenge of low bioavailability for poorly water soluble drug compounds. Am Pharm Rev http://www.americanpharmaceuticalreview.com/Featured-Articles/39299 [Consulted February 11, 2016]. 2012.
MacGregor KJ, Embleton JK, Lacy JE, Perry EA, Solomon LJ, Seager H, et al. Influence of lipolysis on drug absorption from the gastro-intestinal tract. Adv Drug Deliv Rev. 1997;25(1):33–46.
Kahan BD, Dunn J, Fitts C, Van DB, Wombolt D, Pollak R, et al. Reduced inter- and intrasubject variability in cyclosporine pharmacokinetics in renal transplant recipients treated with a microemulsion formulation in conjunction with fasting, low-fat meals, or high-fat meals. Transplantation. 1995;59(4):505–11.
Mendez R, Abboud H, Burdick J, Copley B, Freeman R, Batiuk TD, et al. Reduced intrapatient variability of cyclosporine pharmacokinetics in renal transplant recipients switched from oral Sandimmune to Neoral. Clin Ther. 1999;21(1):160–71.
Katneni K, Charman SA, Porter CJ. Impact of Cremophor-EL and Polysorbate-80 on digoxin permeability across rat jejunum: delineation of thermodynamic and transporter related events using the reciprocal permeability approach. J Pharm Sci. 2007;96(2):280–93.
Kim AE, Dintaman JM, Waddell DS, Silverman JA. Saquinavir, an HIV protease inhibitor, is transported by P-glycoprotein. J Pharmacol Exp Ther. 1998;286(3):1439–45.
Schmitt C, Kaeser B, Riek M, Bech N, Kreuzer C. Effect of saquinavir/ritonavir on P-glycoprotein activity in healthy volunteers using digoxin as a probe. Int J Clin Pharmacol Ther. 2010;48(3):192–9.
Perloff MD, Von Moltke LL, Marchand JE, Greenblatt DJ. Ritonavir induces P-glycoprotein expression, multidrug resistance-associated protein (MRP1) expression, and drug transporter-mediated activity in a human intestinal cell line. J Pharm Sci. 2001;90(11):1829–37.
Chen XQGO, Hageman MJ. Application of lipid-based formulations in drug discovery. J Med Chem. 2012;55:7945–56.
Gao P, Morozowich W. Case studies: rational development of self-emulsifying formulations for improving the oral bioavailability of poorly soluble, lipophilic drugs. Drugs and the Pharmaceutical Sciences. 2007;170:273.
Backman TW, Cao Y, Girke T. ChemMine tools: an online service for analyzing and clustering small molecules. Nucleic Acids Res. 2011;39(suppl_2):W486–W91.
Constantinides PP, Wasan KM. Lipid formulation strategies for enhancing intestinal transport and absorption of P-glycoprotein (P-gp) substrate drugs: in vitro/In vivo case studies. J Pharm Sci. 2007;96(2):235–48.
Aungst BJ. Absorption enhancers: applications and advances. AAPS J. 2012;14(1):10–8.
Akhtar N, Ahad A, Khar RK, Jaggi M, Aqil M, Iqbal Z, et al. The emerging role of P-glycoprotein inhibitors in drug delivery: a patent review. Expert Opin Ther Pat. 2011;21(4):561–76.
Kayser O, Olbrich C, Yardley V, Kiderlen A, Croft S. Formulation of amphotericin B as nanosuspension for oral administration. Int J Pharm. 2003;254(1):73–5.
Lindmark T, Kimura Y, Artursson P. Absorption enhancement through intracellular regulation of tight junction permeability by medium chain fatty acids in Caco-2 cells. J Pharmacol Exp Ther. 1998;284(1):362–9.
Veber DF, Johnson SR, Cheng H-Y, Smith BR, Ward KW, Kopple KD. Molecular properties that influence the oral bioavailability of drug candidates. J Med Chem. 2002;45(12):2615–23.
Flaten GE, Luthman K, Vasskog T, Brandl M. Drug permeability across a phospholipid vesicle-based barrier: 4. The effect of tensides, co-solvents and pH changes on barrier integrity and on drug permeability. Eur J Pharm Sci. 2008;34(2–3):173–80.
Müllertz A, Ogbonna A, Ren S, Rades T. New perspectives on lipid and surfactant based drug delivery systems for oral delivery of poorly soluble drugs. J Pharm Pharmacol. 2010;62(11):1622–36.
Bajusz D, Rácz A, Héberger K. Why is Tanimoto index an appropriate choice for fingerprint-based similarity calculations? J Chem Inf. 2015;7(1):20.
Stuchlík M, Zak S. Lipid-based vehicle for oral drug delivery. Biomed Pap. 2001;145(2):17–26.
Porter CJ, Trevaskis NL, Charman WN. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat Rev Drug Discov. 2007;6(3):231.
Hauss DJ. Oral lipid-based formulations. Adv Drug Deliv Rev. 2007;59(7):667–76.
Hauss DJ. Oral lipid-based formulations: enhancing the bioavailability of poorly water-soluble drugs. CRC Press; 2007.
van Oss CJ. Nonionic surfactants: physical chemistry (surfactant science series Vol. 23). MJ Schick (ed) Marcel Dekker, Inc., New York and Basel, 1987, pp. xv + 1136, $225.00. J Dispers Sci Technol. 1990;11(4):437–8.
Attwood D, Florence A. Surfactant systems—their chemistry, pharmacy and biology. New York: Chapman and Hall; 1983.
Padley FB, Gunstone FD, Harwood JL. Occurrence and characteristics of oils and fats. The lipid handbook. Berlin: Springer; 1986. p. 49–170.
Chen M-L. Lipid excipients and delivery systems for pharmaceutical development: a regulatory perspective. Adv Drug Deliv Rev. 2008;60(6):768–77.
Maincent P. The regulatory environment: the challenges for lipid-based formulation. Bulletin Technique Gattefossé. 2007;100:47–9.
U.S. Food and Drug Administration, Center for Drug Evaluation and Research, Inactive ingredient guide. Division of Drug Information Resources. [Internet]. Office of Management, Center for Drug Evaluation and Research, Food and Drug Administration. 1996. Available from: http://www.fda.gov/cder/drug/iig/default.htm.
U.S. Food and Drug Administration, Title 21, Code of Federal Regulations, Part 182, 184, 186. Office of the Federal Register [Internet]. National Archives and Records Administration. 2007.
Ghosh S, Roy T. Nanoparticulate drug-delivery systems: lymphatic uptake and its gastrointestinal applications. 2014 [cited 4 06]. 123–30].
Guidance for Industry: Nonclinical studies for the safety evaluation of pharmaceutical excipients. Office of Training and Communication, Division of Drug Information, HFD-240, Center for Drug Evaluation and Research, Food and Drug Administration, or Office of Communication, Training, and Manufacturers Assistance, HFM-40, Center for Biologics Evaluation and Research, Food and Drug Administration; 2005.
Mukherjee B, Maji R, Roychowdhury S, Ghosh S. Toxicological concerns of engineered nanosize drug delivery systems. Am J Ther. 2016;23(1):e139–e50.
Szebeni J, Muggia F, Gabizon A, Barenholz Y. Activation of complement by therapeutic liposomes and other lipid excipient-based therapeutic products: prediction and prevention. Adv Drug Deliv Rev. 2011;63(12):1020–30.
Kaukonen AM, Boyd BJ, Porter CJ, Charman WN. Drug solubilization behavior during in vitro digestion of simple triglyceride lipid solution formulations. Pharm Res. 2004;21(2):245–53.
Pouton CW. Formulation of self-emulsifying drug delivery systems. Adv Drug Deliv Rev. 1997;25(1):47–58.
Pouton CW. Self-emulsifying drug delivery systems: assessment of the efficiency of emulsification. Int J Pharm. 1985;27(2–3):335–48.
Rowe RC, Sheskey PJ, Owen SC. Handbook of pharmaceutical excipients. London: Pharmaceutical Press; 2006.
Schick MJ. Nonionic surfactants: physical chemistry. Boca Raton: CRC Press; 1987.
Shono Y, Nishihara H, Matsuda Y, Furukawa S, Okada N, Fujita T, et al. Modulation of intestinal P-glycoprotein function by cremophor EL and other surfactants by an in vitro diffusion chamber method using the isolated rat intestinal membranes. J Pharm Sci. 2004;93(4):877–85.
Hugger ED, Novak BL, Burton PS, Audus KL, Borchardt RT. A comparison of commonly used polyethoxylated pharmaceutical excipients on their ability to inhibit P-glycoprotein activity in vitro. J Pharm Sci. 2002;91(9):1991–2002.
Cole ET, Cadé D, Benameur H. Challenges and opportunities in the encapsulation of liquid and semi-solid formulations into capsules for oral administration. Adv Drug Deliv Rev. 2008;60(6):747–56.
Cullis PR, Hope MJ, Tilcock CP. Lipid polymorphism and the roles of lipids in membranes. Chem Phys Lipids. 1986;40(2–4):127–44.
Hafez IM, Cullis PR. Roles of lipid polymorphism in intracellular delivery. Adv Drug Deliv Rev. 2001;47(2–3):139–48.
Holm R, Porter CJ, Edwards GA, Müllertz A, Kristensen HG, Charman WN. Examination of oral absorption and lymphatic transport of halofantrine in a triple-cannulated canine model after administration in self-microemulsifying drug delivery systems (SMEDDS) containing structured triglycerides. Eur J Pharm Sci. 2003;20(1):91–7.
Cavalli R, Zara GP, Caputo O, Bargoni A, Fundarò A, Gasco MR. Transmucosal transport of tobramycin incorporated in SLN after duodenal administration to rats. Part I—a pharmacokinetic study. Pharmacol Res. 2000;42(6):541–5.
Bargoni A, Cavalli R, Caputo O, Fundarò A, Gasco MR, Zara GP. Solid lipid nanoparticles in lymph and plasma after duodenal administration to rats. Pharm Res. 1998;15(5):745–50.
Battaglia L, Serpe L, Muntoni E, Zara G, Trotta M, Gallarate M. Methotrexate-loaded SLNs prepared by coacervation technique: in vitro cytotoxicity and in vivo pharmacokinetics and biodistribution. Nanomedicine. 2011;6(9):1561–73.
Manjunath K, Venkateswarlu V. Pharmacokinetics, tissue distribution and bioavailability of nitrendipine solid lipid nanoparticles after intravenous and intraduodenal administration. J Drug Target. 2006;14(9):632–45.
Makwana V, Jain R, Patel K, Nivsarkar M, Joshi A. Solid lipid nanoparticles (SLN) of Efavirenz as lymph targeting drug delivery system: elucidation of mechanism of uptake using chylomicron flow blocking approach. Int J Pharm. 2015;495(1):439–46.
Shete H, Chatterjee S, De A, Patravale V. Long chain lipid based tamoxifen NLC. Part II: pharmacokinetic, biodistribution and in vitro anticancer efficacy studies. Int J Pharm. 2013;454(1):584–92.
Zhang T, Chen J, Zhang Y, Shen Q, Pan W. Characterization and evaluation of nanostructured lipid carrier as a vehicle for oral delivery of etoposide. Eur J Pharm Sci. 2011;43(3):174–9.
Myers R, Stella V. Factors affecting the lymphatic transport of penclomedine (NSC-338720), a lipophilic cytotoxic drug: comparison to DDT and hexachlorobenzene. Int J Pharm. 1992;80(1–3):51–62.
Gursoy RN, Benita S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed Pharmacother. 2004;58(3):173–82.
Woo JS, Kim T-S, Park J-H, Chi S-C. Formulation and biopharmaceutical evaluation of silymarin using SMEDDS. Arch Pharm Res. 2007;30(1):82–9.
Khumpirapang N, Pikulkaew S, Müllertz A, Rades T, Okonogi S. Self-microemulsifying drug delivery system and nanoemulsion for enhancing aqueous miscibility of Alpinia galanga oil. PloS One. 2017;12(11):e0188848.
Thakkar H, Nangesh J, Parmar M, Patel D. Formulation and characterization of lipid-based drug delivery system of raloxifene-microemulsion and self-microemulsifying drug delivery system. J Pharm Bioallied Sci. 2011;3(3):442.
Chen Y, Li G, Wu X, Chen Z, Hang J, Qin B, et al. Self-microemulsifying drug delivery system (SMEDDS) of vinpocetine: formulation development and in vivo assessment. Biol Pharm Bull. 2008;31(1):118–25.
Nishioka Y, Yoshino H. Lymphatic targeting with nanoparticulate system. Adv Drug Deliv Rev. 2001;47(1):55–64.
Sheue Nee Ling S, Magosso E, Abdul Karim Khan N, Hay Yuen K, Anne Barker S. Enhanced oral bioavailability and intestinal lymphatic transport of a hydrophilic drug using liposomes. Drug Dev Ind Pharm. 2006;32(3):335–45.
Perrie Y, Obrenovic M, McCarthy D, Gregoriadis G. Liposome (Lipodine™)-mediated DNA vaccination by the oral route. Journal of Liposome Research. 2002;12(1–2):185–97.
Li H, Song J-H, Park J-S, Han K. Polyethylene glycol-coated liposomes for oral delivery of recombinant human epidermal growth factor. Int J Pharm. 2003;258(1–2):11–9.
Larsen DB, Joergensen S, Olsen NV, Hansen SH, Larsen C. In vivo release of bupivacaine from subcutaneously administered oily solution. Comparison with in vitro release. J Control Release. 2002;81(1–2):145–54.
Gill KK, Kaddoumi A, Nazzal S. Mixed micelles of PEG2000-DSPE and vitamin-E TPGS for concurrent delivery of paclitaxel and parthenolide: enhanced chemosenstization and antitumor efficacy against non-small cell lung cancer (NSCLC) cell lines. Eur J Pharm Sci. 2012;46(1–2):64–71.
Passerini N, Albertini B, Perissutti B, Rodriguez L. Evaluation of melt granulation and ultrasonic spray congealing as techniques to enhance the dissolution of praziquantel. Int J Pharm. 2006;318(1–2):92–102.
Chauhan B, Shimpi S, Paradkar A. Preparation and evaluation of glibenclamide-polyglycolized glycerides solid dispersions with silicon dioxide by spray drying technique. Eur J Pharm Sci. 2005;26(2):219–30.
Chauhan B, Shimpi S, Paradkar A. Preparation and characterization of etoricoxib solid dispersions using lipid carriers by spray drying technique. AAPS PharmSciTech. 2005;6(3):E405–E9.
Sethia S, Squillante E. Solid dispersion of carbamazepine in PVP K30 by conventional solvent evaporation and supercritical methods. Int J Pharm. 2004;272(1–2):1–10.
Chen C, Han D, Cai C, Tang X. An overview of liposome lyophilization and its future potential. J Control Release. 2010;142(3):299–311.
Dening TJ, Rao S, Thomas N, Prestidge CA. Novel nanostructured solid materials for modulating oral drug delivery from solid-state lipid-based drug delivery systems. AAPS J. 2016;18(1):23–40.
Porter CJ, Charman WN. Model systems for intestinal lymphatic transport studies. Models for assessing drug absorption and metabolism. Springer; 1996. p. 85–102.
Griffin BT, O’Driscoll CM. A comparison of intestinal lymphatic transport and systemic bioavailability of saquinavir from three lipid-based formulations in the anaesthetised rat model. J Pharm Pharmacol. 2006;58(7):917–25.
Perry CM, Noble S. Saquinavir soft-gel capsule formulation. Drugs. 1998;55(3):461–86.
Dahan A, Hoffman A. The effect of different lipid based formulations on the oral absorption of lipophilic drugs: the ability of in vitro lipolysis and consecutive ex vivo intestinal permeability data to predict in vivo bioavailability in rats. Eur J Pharm Biopharm. 2007;67(1):96–105.
Hauss DJ, Fogal SE, Ficorilli Ficorilli JV. Chronic collection of mesenteric lymph from conscious, tethered rats. J Am Assoc Lab Anim Sci. 1998;37(3):56–8.
Trevaskis NL, Hu L, Caliph SM, Han S, Porter CJ. The mesenteric lymph duct cannulated rat model: application to the assessment of intestinal lymphatic drug transport. J Vis Exp. 2015;97.
Chen G-L, Hao W-H. Factors affecting zero-order release kinetics of porous gelatin capsules. Drug Dev Ind Pharm. 1998;24(6):557–62.
Kim Y-H, Koczo K, Wasan DT. Dynamic film and interfacial tensions in emulsion and foam systems. J Colloid Interface Sci. 1997;187(1):29–44.
Yang S, Simmonds RS, Birch EJ. Physicochemical characterization and thermal properties of lipids from R. opacus PD630. Food and Public Health. 2014;4(3):87–92.
Shah BR, Finberg L. Single-day therapy for nutritional vitamin D-deficiency rickets: a preferred method. J Pediatr. 1994;125(3):487–90.
Yuan T, Qin L, Wang Z, Nie J, Guo Z, Li G, et al. Solid lipid dispersion of calcitriol with enhanced dissolution and stability. AJPS. 2013;8(1):39–47.
Singh A, Narsipur S. Cyclosporine: a commentary on brand versus generic formulation exchange. J Trans. 2011;2011.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Guest Editor: Sanyog Jain
Rights and permissions
About this article
Cite this article
Patel, V., Lalani, R., Bardoliwala, D. et al. Lipid-Based Oral Formulation Strategies for Lipophilic Drugs. AAPS PharmSciTech 19, 3609–3630 (2018). https://doi.org/10.1208/s12249-018-1188-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-018-1188-8