Abstract
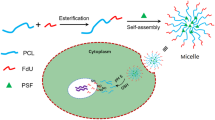
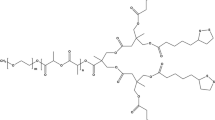
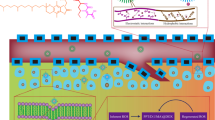
The complex design of multifunctional nanomedicine is beneficial to overcome the multiple biological barriers of drug delivery, but it also presents additional hurdles to clinical translation (e.g., scaling-up and quality control). To address this dilemma, we employed a simple imidazole-bearing polymer micelle for enhanced cellular uptake, facilitated endosomal escape, and on-demand release of a model drug, SN-38. The micelles were crosslinked by the reversible imidazole/Zn2+ coordination with a drug loading of ca. 4% (w/w) and a diameter less than 200 nm. Under mimicked tumor microenvironment (pH 6.8), the surface charge of micelles reversed from negative to positive, leading to enhanced micelles uptake by model 4T1 cells. Such effect was verified by fluorescent labelling of micelles. Compared to imidazole-free nanocarriers, the charge-reversal micelles delivered significantly more SN-38 to 4T1 cells. Due to the proton sponge effect, imidazole-bearing micelles could rapidly escape from endosomes compared to the control micelles, as evidenced by the kinetic analysis of micelle/endosome co-localization. The coordination crosslinking also enabled the acid-triggered drug release. This work provides a “three birds with one stone” approach to achieve the multifunctionality of nanocarriers without complicated particle design, and opens new avenues of advancing nanomedicine translation via simple tailored nanocarriers.









Similar content being viewed by others
References
Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–60.
Min Y, Caster JM, Eblan MJ, Wang AZ. Clinical translation of nanomedicine. Chem Rev. 2015;115:11147–90.
Estanqueiro M, Amaral MH, Conceicao J, Sousa Lobo JM. Nanotechnological carriers for cancer chemotherapy: the state of the art. Colloids Surf B Biointerfaces. 2015;126:631–48.
Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33:941–51.
Cheng Z, A Zaki A, Hui JZ, Muzykantov VR, Tsourkas A. Multifunctional nanoparticles: cost versus benefit of adding targeting and imaging capabilities. Science 2012; 338:903–910.
Zhang Z, Yin L, Tu C, Song Z, Zhang Y, Xu Y, et al. Redox-responsive, core cross-linked polyester micelles. ACS Macro Lett. 2013;2:40–4.
Torchilin VP. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat Rev Drug Discov. 2014;13:813–27.
Lammers T, Aime S, Hennink WE, Storm G, Kiessling F. Theranostic nanomedicine. Acc Chem Res. 2011;44:1029–38.
Cha R, Li J, Liu Y, Zhang Y, Xie Q, Zhang M. Fe3O4 nanoparticles modified by CD-containing star polymer for MRI and drug delivery. Colloids Surf B Biointerfaces. 2017;158:213–21.
Barenholz Y, Peer D. Liposomes and other assemblies as drugs and nano-drugs: from basic and translational research to the clinics. J Control Release. 2012;160:115–6.
Louage B, De Wever O, Hennink WE, De Geest BG. Developments and future clinical outlook of taxane nanomedicines. J Control Release. 2017;253:137–52.
Bauman JE, Wang JC. Imidazole complexes of nickel(II), copper(II), zinc(II), and silver(I). Inorg Chem. 1964;3:368–73.
Yu C, Gao C, Lü S, Chen C, Yang J, Di X, et al. Facile preparation of pH-sensitive micelles self-assembled from amphiphilic chondroitin sulfate-histamine conjugate for triggered intracellular drug release. Colloids Surf B Biointerfaces. 2014;115:331–9.
Li X, Gao M, Xin K, Zhang L, Ding D, Kong D, et al. Singlet oxygen-responsive micelles for enhanced photodynamic therapy. J Control Release. 2017;260:12–21.
Wu H, Zhu L, Torchilin VP. pH-sensitive poly(histidine)-PEG/DSPE-PEG co-polymer micelles for cytosolic drug delivery. Biomaterials. 2013;34:1213–22.
Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci U S A. 1995;92:7297–301.
Nel AE, Madler L, Velegol D, Xia T, Hoek EMV, Somasundaran P, et al. Understanding bio-physicochemical interactions at the nano-bio interface. Nat Mater. 2009;8:543–57.
Xin K, Li M, Lu D, Meng X, Deng J, Kong D, et al. Bioinspired coordination micelles integrating high stability, triggered cargo release, and magnetic resonance imaging. ACS Appl Mater Interfaces. 2017;9:80–91.
Cabral H, Nishiyama N, Kataoka K. Supramolecular nanodevices: from design validation to theranostic nanomedicine. Acc Chem Res. 2011;44:999–1008.
Zhao CL, Winnik MA, Riess G, Croucher MD. Fluorescence probe techniques used to study micelle formation in water-soluble block copolymers. Langmuir. 1990;6:514–6.
Yang R, Zhang S, Kong D, Gao X, Zhao Y, Wang Z. Biodegradable polymer-curcumin conjugate micelles enhance the loading and delivery of low-potency curcumin. Pharm Res. 2012;29:3512–25.
Huang Y, Tang Z, Zhang X, Yu H, Sun H, Pang X, et al. pH-triggered charge-reversal polypeptide nanoparticles for cisplatin delivery: preparation and in vitro evaluation. Biomacromolecules. 2013;14:2023–32.
Yue Y, Jin F, Deng R, Cai J, Dai Z, Lin MC, et al. Revisit complexation between DNA and polyethylenimine-effect of length of free polycationic chains on gene transfection. J Control Release. 2011;152:143–51.
Shi J, Schellinger JG, Johnson RN, Choi JL, Chou B, Anghel EL, et al. Influence of histidine incorporation on buffer capacity and gene transfection efficiency of HPMA-co-oligolysine brush polymers. Biomacromolecules. 2013;2013(14):1961–70.
Tabushi I, Kuroda Y. Bis(histamino) cyclodextin-zn-imidazole complex as an artificial carbonic anhydrase. J Am Chem Soc. 1984;15:4580–4.
Deng J, Wang K, Wang M, Yu P, Mao L. Mitochondria targeted nanoscale zeolitic imidazole framework-90 (ZIF-90) for ATP imaging in live cells. J Am Chem Soc. 2017;139:5877–82.
Anderson EB, Long TE. Imidazole- and imidazolium-containing polymers for biology and material science applications. Polymer. 2010;51:2447–54.
Torchilin VP. Micellar nanocarriers: pharmaceutical perspectives. Pharm Res. 2007;24:1–16.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation cell. Cell. 2011;144:646–74.
Han SS, Li ZY, Zhu JY, Han K, Zeng ZY, Hong W, et al. Dual-pH sensitive charge-reversal polypeptide micelles for tumor-triggered targeting uptake and nuclear drug delivery. Small. 2015;11:2543–54.
Wang Z, Chen C, Liu R, Fan A, Kong D, Zhao Y. Two birds with one stone: dendrimer surface engineering enables tunable periphery hydrophobicity and rapid endosomal escape. Chem Commun. 2014;50:14025–8.
Mok H, Park JW, Park TG. Enhanced intracellular delivery of quantum dot and adenovirus nanoparticles triggered by acidic pH via surface charge reversal. Bioconjug Chem. 2008;19:797–801.
Shen Y, Zhou Z, Sui M, Tang J, Xu P, Van Kirk EA, et al. Charge-reversal polyamidoamine dendrimer for cascade nuclear drug delivery. Nanomedicine. 2010;5:1205–17.
Chen C, Tao R, Ding D, Kong D, Fan A, Wang Z, et al. Ratiometric co-delivery of multiple chemodrugs in a single nanocarrier. Eur J Pharm Sci. 2017;107:16–23.
Varkouhi AK, Scholte M, Storm G, Haisma HJ. Endosomal escape pathways for delivery of biologicals. J Control Release. 2011;151:220–8.
French AP, Mills S, Swarup R, Bennett MJ, Pridmore TP. Colocalization of fluorescent markers in confocal microscope images of plant cells. Nat Protoc. 2008;3:619–28.
Funding
The work was financially supported by the National Natural Science Foundation of China (21650110447), State Key Laboratory of Medicinal Chemical Biology (Nankai University) (2017030), and the innovation fund of Tianjin University (1706).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
ESM 1
(DOC 7292 kb)
Rights and permissions
About this article
Cite this article
Lu, D., An, Y., Feng, S. et al. Imidazole-Bearing Polymeric Micelles for Enhanced Cellular Uptake, Rapid Endosomal Escape, and On-demand Cargo Release. AAPS PharmSciTech 19, 2610–2619 (2018). https://doi.org/10.1208/s12249-018-1092-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-018-1092-2