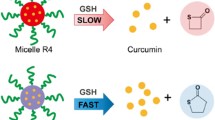
ABSTRACT
Purpose
To utilize a novel type of polymer-drug conjugate micelle to enhance the delivery of low-potency curcumin.
Methods
Multiple curcumin molecules were conjugated to poly(lactic acid) (PLA) via tris(hydroxymethyl)aminomethane (Tris) linker producing the hydrophobic drug-binding block; methoxy-poly(ethylene glycol) (mPEG) was employed as the hydrophilic block. Micelles were characterized by size, loading capacity, stability, and critical micelle concentration (CMC). Human hepatocellular carcinoma (HepG2) cells were employed to assess cytotoxicity and intracellular targeting ability of micelles.
Results
mPEG-PLA-Tris-Cur micelles were within nanorange (<100 nm). CMC of such micelles (2.3 ± 0.4 μg/mL) was 10 times lower than mPEG-PLA micelles (27.4 ± 0.8 μg/mL). Curcumin loading in mPEG-PLA-Tris-Cur micelles reached 18.5 ± 1.3% (w/w), compared to traditional mPEG-PLA micelles at 3.6 ± 0.4% (w/w). IC50 of mPEG-PLA-Tris-Cur micelles (~22 μg/mL at curcumin-equivalent dose) was similar to unmodified curcumin. Placebo and drug-encapsulated conjugate micelles could be efficiently internalized to cytoplasmic compartment of HepG2 cells.
Conclusions
Micelle-forming polymer-drug conjugates containing multiple drug molecules were an efficient means to increase loading and intracellular delivery of low-potency curcumin.









Similar content being viewed by others
Abbreviations
- CLSM:
-
confocal laser scanning microscope
- CMC:
-
critical micelle concentration
- Cur:
-
curcumin
- Cur-GA:
-
mono-carboxyl-terminated curcumin
- DCC:
-
dicyclohexylcarbodiimide
- DMAP:
-
4-dimethylamino pyridine
- DMEM:
-
Dulbecco’s modification of eagle’s medium
- DMF:
-
N,N-dimethylformamide
- EPR:
-
enhanced permeability and retention effect
- GA:
-
gluraric anhydride
- GRAS:
-
generally regarded as safe
- HepG2:
-
human hepatocellular carcinoma cells
- HPLC:
-
high performance liquid chromatography
- mPEG:
-
methoxy-poly(ethylene glycol)
- MPS:
-
mononuclear phagocyte system
- MWCO:
-
molecular weight cut-off
- NHS:
-
N-hydroxy succinimide
- NMR:
-
nuclear magnetic resonance
- PCL:
-
polycaprolactone
- PLA:
-
poly(lactic acid)
- RBC:
-
red blood cells
- THF:
-
tetrahydrofuran
- Tris:
-
tris(hydroxymethyl)aminomethane
References
Shen Y, Jin E, Zhang B, Murphy CJ, Sui M, Zhao J, Wang J, Tang J, Fan M, Van Kirk E, Murdoch WJ. Prodrugs forming high drug loading multifunctional nanocapsules for intracellular cancer drug delivery. J Am Chem Soc. 2010;132(12):4259–65.
Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005;5(3):161–71.
Shin HC, Alani AWG, Cho H, Bae Y, Kolesar JM, Kwon GS. A 3-in-1 Polymeric Micelle nanocontainer for poorly water-soluble drugs. Mol Pharm. 2011;8(4):1257–65.
Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2(12):751–60.
Tang H, Murphy CJ, Zhang B, Shen Y, Sui M, Van Kirk EA, Feng X, Murdoch W. Amphiphilic curcumin conjugate-forming nanoparticles as anticancer prodrug and drug carriers: in vitro and in vivo effects. Nanomedicine (Lond). 2010;5(6):855–65.
Tang H, Murphy CJ, Zhang B, Shen Y, Van Kirk EA, Murdoch WJ, Radosz M. Curcumin polymers as anticancer conjugates. Biomaterials. 2010;31(27):7139–49.
Lao C, Ruffin M, Normolle D, Heath D, Murray S, Bailey J, Boggs M, Crowell J, Rock C, Brenner D. Dose escalation of a curcuminoid formulation. BMC Complementary Altern Med. 2006;6(1):10.
Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4(6):807–18.
Yallapu MM, Jaggi M, Chauhan SC. Beta-cyclodextrin-curcumin self-assembly enhances curcumin delivery in prostate cancer cells. Colloids Surf B. 2010;79(1):113–25.
Anand P, Nair HB, Sung BK, Kunnumakkara AB, Yadav VR, Tekmal RR, Aggarwal BB. Design of curcumin-loaded PLGA nanoparticles formulation with enhanced cellular uptake, and increased bioactivity in vitro and superior bioavailability in vivo. Biochem Pharmacol. 2010;79(3):330–8.
Shahani K, Panyam J. Highly loaded, sustained-release microparticles of curcumin for chemoprevention. J Pharm Sci. 2011;100(7):2599–609.
Safavy A, Raisch KP, Mantena S, Sanford LL, Sham SW, Krishna NR, Bonner JA. Design and development of water-soluble curcumin conjugates as potential anticancer agents. J Med Chem. 2007;50(24):6284–8.
Zhang F, Koh GY, Jeansonne DP, Hollingsworth J, Russo PS, Vicente G, Stout RW, Liu Z. A novel solubility-enhanced curcumin formulation showing stability and maintenance of anticancer activity. J Pharm Sci. 2011;100(7):2778–89.
Manju S, Sreenivasan K. Conjugation of curcumin onto hyaluronic acid enhances its aqueous solubility and stability. J Colloid Interface Sci. 2011;359(1):318–25.
Manju S, Sreenivasan K. Synthesis and characterization of a cytotoxic cationic polyvinylpyrrolidone-curcumin conjugate. J Pharm Sci. 2011;100(2):504–11.
Ma Z, Haddadi A, Molavi O, Lavasanifar A, Lai R, Samuel J. Micelles of poly(ethylene oxide)-b-poly(epsilon-caprolactone) as vehicles for the solubilization, stabilization, and controlled delivery of curcumin. J Biomed Mater Res A. 2008;86(2):300–10.
Gou M, Men K, Shi H, Xiang M, Zhang J, Song J, Long J, Wan Y, Luo F, Zhao X, Qian Z. Curcumin-loaded biodegradable polymeric micelles for colon cancer therapy in vitro and in vivo. Nanoscale. 2011;3(4):1558–67.
Mohanty C, Acharya S, Mohanty AK, Dilnawaz F, Sahoo SK. Curcumin-encapsulated MePEG/PCL diblock copolymeric micelles: a novel controlled delivery vehicle for cancer therapy. Nanomedicine (Lond). 2010;5(3):433–49.
Pitarresi G, Palumbo FS, Albanese A, Fiorica C, Picone P, Giammona G. Self-assembled amphiphilic hyaluronic acid graft copolymers for targeted release of antitumoral drug. J Drug Target. 2010;18(4):264–76.
Sahu A, Bora U, Kasoju N, Goswami P. Synthesis of novel biodegradable and self-assembling methoxy poly(ethylene glycol)-palmitate nanocarrier for curcumin delivery to cancer cells. Acta Biomater. 2008;4(6):1752–61.
Li ZB, Kesselman E, Talmon Y, Hillmyer MA, Lodge TP. Multicompartment micelles from ABC miktoarm stars in water. Science. 2004;306(5693):98–101.
Yokoyama M, Kwon GS, Okano T, Sakurai Y, Seto T, Kataoka K. Preparation of micelle-forming polymer-drug conjugates. Bioconjugate Chem. 1992;3(4):295–301.
Hu X, Jing X. Biodegradable amphiphilic polymer-drug conjugate micelles. Expert Opin Drug Deliv. 2009;6(10):1079–90.
Discher DE, Kamien RD. Self-assembly —towards precision micelles. Nature. 2004;430(6999):519–20.
Hans M, Shimoni K, Danino D, Siegel SJ, Lowman A. Synthesis and characterization of mPEG-PLA prodrug micelles. Biomacromolecules. 2005;6(5):2708–17.
Shi W, Dolai S, Rizk S, Hussain A, Tariq H, Averick S, L’Amoreaux W, El Idrissi A, Banerjee P, Raja K. Synthesis of monofunctional curcumin derivatives, clicked curcumin dimer, and a PAMAM dendrimer curcumin conjugate for therapeutic applications. Org Lett. 2007;9(26):5461–4.
Li S, Vert M. Synthesis, characterization, and stereocomplex-induced gelation of block copolymers prepared by ring-opening polymerization of l(d)-lactide in the presence of poly(ethylene glycol). Macromolecules. 2003;36(21):8008–14.
Zhang X, Li Y, Chen X, Wang X, Xu X, Liang Q, Hu J, Jing X. Synthesis and characterization of the paclitaxel/MPEG-PLA block copolymer conjugate. Biomaterials. 2005;26(14):2121–8.
Zhu W, Li Y, Liu L, Zhang W, Chen Y, Xi F. Biamphiphilic triblock copolymer micelles as a multifunctional platform for anticancer drug delivery. J Biomed Mater Res A. 2011;96A(2):330–40.
Date AA, Nagarsenker MS, Patere S, Dhawan V, Gude RP, Hassan PA, Aswal V, Steiniger F, Thamm J, Fahr A. Lecithin-based novel cationic nanocarriers (Leciplex) II: improving therapeutic efficacy of quercetin on oral administration. Mol Pharm. 2011;8(3):716–26.
Prajapati RN, Tekade RK, Gupta U, Gajbhiye V, Jain NK. Dendimer-mediated solubilization, formulation development and in vitro-in vivo assessment of piroxicam. Mol Pharm. 2009;6(3):940–50.
Kwon GS, Okano T. Soluble Self-assembled block copolymers for drug delivery. Pharm Res. 1999;16(5):597–600.
Klibanov AL, Maruyama K, Torchilin VP, Huang L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990;268(1):235–7.
Torchilin V. Micellar nanocarriers: pharmaceutical perspectives. Pharm Res. 2007;24(1):1–16.
Zhang L, Eisenberg A. Multiple morphologies of “crew-cut” aggregates of polystyrene-b-poly(acrylic acid) block copolymers. Science. 1995;268(5218):1728–31.
Tobio M, Gref R, Sanchez A, Langer R, Alonso MJ. Stealth PLA-PEG nanoparticles as protein carriers for nasal administration. Pharm Res. 1998;15(2):270–5.
Parikh T, Bommana MM, Squillante III E. Efficacy of surface charge in targeting pegylated nanoparticles of sulpiride to the brain. Eur J Pharm Biopharm. 2010;74(3):442–50.
Dong Y, Feng SS. Methoxy poly(ethylene glycol)-poly(lactide) (MPEG-PLA) nanoparticles for controlled delivery of anticancer drugs. Biomaterials. 2004;25(14):2843–9.
Gref R, Miralles G, Dellacherie E. Polyoxyethylene-coated nanospheres: effect of coating on zeta potential and phagocytosis. Polym Int. 1999;48(4):251–6.
Lee J, Cho EC, Cho K. Incorporation and release behavior of hydrophobic drug in functionalized poly(d, l-lactide)-block-poly(ethylene oxide) micelles. J Control Release. 2004;94(2–3):323–35.
Allen C, Maysinger D, Eisenberg A. Nano-engineering block copolymer aggregates for drug delivery. Colloids Surf B. 1999;16(1–4):3–27.
Nagarajan R, Ganesh K. Block copolymer self-assembly in selective solvents: theory of solubilization in spherical micelles. Macromolecules. 1989;22(11):4312–25.
Kim SH, Tan JPK, Nederberg F, Fukushima K, Colson J, Yang C, Nelson A, Yang YY, Hedrick JL. Hydrogen bonding-enhanced micelle assemblies for drug delivery. Biomaterials. 2010;31(31):8063–71.
Lee ALZ, Venkataraman S, Sirat SBM, Gao S, Hedrick JL, Yang YY. The use of cholesterol-containing biodegradable block copolymers to exploit hydrophobic interactions for the delivery of anticancer drugs. Biomaterials. 2012;33(6):1921–8.
Wang YJ, Pan MH, Cheng AL, Lin LI, Ho YS, Hsieh CY, Lin JK. Stability of curcumin in buffer solutions and characterization of its degradation products. J Pharm Biomed Anal. 1997;15(12):1867–76.
Yallapu MM, Ebeling MC, Chauhan N, Jaggi M, Chauhan SC. Interaction of curcumin nanoformulations with human plasma proteins and erythrocytes. Int J Nanomedicine. 2011;6:2779–90.
Shuai X, Ai H, Nasongkla N, Kim S, Gao J. Micellar carriers based on block copolymers of poly(epsilon-caprolactone) and poly(ethylene glycol) for doxorubicin delivery. J Control Release. 2004;98(3):415–26.
Luo L, Tam J, Maysinger D, Eisenberg A. Cellular internalization of poly(ethylene oxide)-b-poly(ε-caprolactone) diblock copolymer micelles. Bioconjugate Chem. 2002;13(6):1259–65.
Aryal S, Hu CMJ, Zhang L. Polymeric nanoparticles with precise ratiometric control over drug loading for combination therapy. Mol Pharm. 2011;8(4):1401–7.
Savic R, Luo L, Eisenberg A, Maysinger D. Micellar nanocontainers distribute to defined cytoplasmic organelles. Science. 2003;300(5619):615–8.
Acknowledgments and Disclosures
This work was supported by Tianjin Research Program of Application Foundation and Advanced Technology (11JCZDJC20600; 11JCYBJC10300), National Natural Science Foundation of China (81171478; 31100699), and the Research Fund for the Doctoral Program of Higher Education of China (20110032120077).
The authors of this article have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yang, R., Zhang, S., Kong, D. et al. Biodegradable Polymer-Curcumin Conjugate Micelles Enhance the Loading and Delivery of Low-Potency Curcumin. Pharm Res 29, 3512–3525 (2012). https://doi.org/10.1007/s11095-012-0848-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-012-0848-8