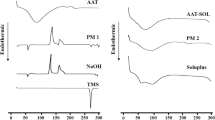
ABSTRACT
Sertraline hydrochloride has low solubility and undergoes first-pass metabolism resulting in low bioavailability. The main objective of this research was to enhance the dissolution rate of the drug. The drug was recrystallized in the presence of polymers and surfactant. The formulation was optimized by studying the effects of drug/polymer ratio, concentration of SLS, and type of polymer on particle size and drug release. The optimized formulation was characterized using different techniques and by evaluating in vitro release, stability, and flow properties. A tablet was compressed and evaluated for hardness, friability, and in vitro dissolution. Release profile of the drug from the optimum formulation (poloxamer 407, drug/polymer ratio 1:2/3, and 0.05% SLS) was higher (96%) than that from processed drug alone (56%). After storage of the optimum formulation for 6 months in a desiccator containing silica gel at room temperature, the drug remained crystalline and did not interact with additives, and almost the same cumulative amount (%) of the drug was released as compared to that from the freshly prepared formulation. Flow proprieties were slightly improved. Compressed tablets exhibited acceptable hardness and friability, and the release profile was better (faster and higher) than that from commercial tablet (Zoloft®). In conclusion, the optimum formulation was successful in enhancing the dissolution.











Similar content being viewed by others
REFERENCES
Sertraline FDA Label. http://www.fda.gov/ohrms/dockets/ac/04/briefing/4006b1_06_zoloft-label.pdf. Accessed 2 June 2016.
Flament MF, Lane RM, Zhu R, Ying Z. Predictors of an acute antidepressant response to fluoxetine and sertraline. Int Clin Psychopharmacol. 1999;14:259–75.
Hirschfeld RM. Sertraline in the treatment of anxiety disorders. Depress Anxiety. 2000;11:139–57.
Carrascoa JL, Dı́az-Marsáa M, Sáiz-Ruizb J. Sertraline in the treatment of mixed anxiety and depression disorder. J Affect Disord. 2000;59:67–9.
Watts BV, Schnurr PP, Mayo L, Young-Xu Y, Weeks WB, Friedman MJ. Meta-analysis of the efficacy of treatments for posttraumatic stress disorder. J Clin Psychiatry. 2013;74:541–50.
Passos JJ, De Sousa FB, Lula IS, Barreto EA, Lopes JF, De Almeida WB, et al. Multi-equilibrium system based on sertraline and β-cyclodextrin supramolecular complex in aqueous solution. Int J Pharm. 2011;421:24–33.
Warrier D, Zagade A, Shaikh A, Pawar Y, Kumbhar S. An in-vitro evaluation for the effect of B-cyclodextrin and PVP K-30 on drug release pattern of sertraline hydrochloride. Int J Pharm Chem Sci (IJPCS). 2012;1:407–13.
Kumar A, Sharma P, Chaturvedi A, Jaiswal D, Bajpai M, Choudhary M, et al. Formulation development of sertraline hydrochloride microemulsion for intranasal delivery. Int J ChemTech Res. 2009;1:941–7.
Wagh MP, Patel JS. Biopharmaceutical classification system: scientific basis for biowaiver extensions. Int J Pharm Pharm Sci. 2010;2:12–9.
Pawar HA, Ayre AP, Lalitha KG. Development of validated analytical method for in-vitro dissolution study of sertraline hydrochloride capsules. Curr Pharma Res. 2012;2:560–5.
De Vane CL, Liston HL, Markowitz JS. Clinical pharmacokinetics of sertraline. Clin Pharmacokinet. 2002;41:1247–66.
Kakade SM, Mannur VS, Kardi RV, Ramani KB, Dhada AA. Formulation and evaluation of orally disintegrating tablets of sertraline. Int J Pharma Res Dev-online (IJPRD). 2010;1:1–7.
Patil SK, Wagh KS, Parik VB, Akarte AM, Baviskar DT. Strategies for solubility enhancement of poorly soluble drugs. Int J Pharm Sci Rev Res (IJPSRR). 2011;8:74–80.
Parve B, Shinde P, Rawat S, Rathod S, Waghmode G. Solubility enhancement techniques: a review. World J Pharm Pharm Sci (WJPPS). 2014;3:400–22.
Mehta M, Bhagwat DP, Gupta GD. Fast dissolving tablets of sertraline hydrochloride. Int J ChemTech Res. 2009;1:925–30.
Ammar HO, Ghorab MM, Mostafa DM, Ghoneim AM. Self-Nanoemulsifying drug delivery system for sertraline hydrochloride: design, preparation and characterization. Int J Pharm Pharm Sci. 2014;6:589–95.
Crystallisation Techniques http://depts.washington.edu/eooptic/linkfiles/Crystallisation_Techniques.doc. Accessed 2 June 2016.
Morissette SL, Almarsson O, Peterson ML, Remenar JF, Read MJ, Lemmo AV, et al. High-throughput crystallization: polymorphs, salts, co-crystals and solvates of pharmaceutical solids. Adv Drug Deliv Rev. 2004;56:275–300.
Hammouda YE, El-Khordagui LK, Darwish IA, El-Kamel AH. Manipulation of powder characteristics by interactions at the solid–liquid interface: 1-sulphadiazine. Eur J Pharm Sci. 1999;8:283–90.
Tran TT, Tran PH, Park J, Lee B. Effects of solvents and crystallization conditions on the polymorphic behaviors and dissolution rates of valsartan. Arch Pharm Res. 2012;35:1223–30.
Assaf SM, Khanfar MS, Obeidat R, Salem MS, Arida AI. Effect of different organic solvents on crystal habit of mefenamic acid. Jordan J Pharm Sci (JJPS). 2009;2:150–8.
Garekani HA, Sadeghi F, Badiee A, Mostafa SA, Rajabi-Siahboomi AR. Crystal habit modifications of ibuprofen and their physicmechanical characteristics. Drug Dev Ind Pharm. 2001;27:803–9.
Stieger N, Liebenberg W. Recrystallization of active pharmaceutical ingredients. In: Andreeta M, editor. Crystallization—Science and Technology. Croatia: INTECH Science, Technology and Medicine open access publisher; 2012. p. 187–204 doi:10.5772/52725.
European Pharmacopeia. General Notices (1) 2440–2441.
Kadian SS, Harikumar SL. Eudragit and its pharmaceutical significance. http://www.researchgate.net/publication/228097715_Eudragit_and_its_Pharmaceutical_Significance. Accessed 2 June 2016.
Haaf F, Sanner A, Straub F. Polymers of N-vinylpyrrolidone: synthesis, characterization and uses. Polym J. 1985;17:143–52.
Al-Taani B, Salem MS, Al Taani S. Influence of polyvinyl pyrrolidone addition during crystallization on the physicochemical properties of mefenamic acid crystals. Jordan J Pharm Sci (JJPS). 2009;2:86–98.
Folttmann H, Quadir A. Polyvinylpyrrolidone (PVP)—one of the most widely used excipients in pharmaceuticals: an overview. Drug Deliv Technol. 2008;8:7–22.
Chen J, Spear SK, Huddleston J, Rogers RD. Polyethylene glycol and solutions of polyethylene glycol as green reaction media. Green Chem. 2005;7:64–82.
Almeida H, Amaral MH, Lobão P, Lobo JMS. Pluronic F-127 and pluronic lecithin organogel (PLO): main features and their applications in topical and transdermal administration of drugs. J Pharm Pharm Sci. 2012;15:592–605.
Gohel MC, Sarvaiya KG, Mehta NR, Soni CD, Vyas VU, Dave RK. Assessment of similarity factor using different weighting approaches. Dissolution Technol. 2005;22–27.
A process for making sertraline hydrochloride form II. http://www.allindianpatents.com/patents/236068-a-process-for-making-sertraline-hydrochloride-form-II. Accessed 2 June 2016.
Khamar BM, Modi IA, Rajappa M, Shashikala KN, Achanath R, Chheda A. Process for the preparation of sertraline hydrochloride form II. Patent WO/2006/027658A2
Schwartz E, Nidam T, Liberman A, Mendelovici M, Aronhime J, Singer C, et al. Sertraline hydrochloride polymorphs. Patent US 6,500,987 B1
Sinko PJ. Martin’s physical pharmacy and pharmaceutical sciences. 5th ed. Baltimore: Lippincott Williams and Wilkins; 2006.
EUDRAGIT® L 100 and EUDRAGIT® S 100 technical information issued by Evonik.
Chadha R, Kapoor VK, Kumar A. Analytical techniques used to characterize drug-polyvinylpyrrolidone systems in solid and liquid states-an overview. J Sci Ind Res (JSIR). 2006;65:459–69.
Somasundaran P, Markovic B, Krishnakumar S, Yu X. Colloid systems and interfaces stability of dispersions through polymer and surfactant adsorption. In: Birdi KS, editor. Handbook of surface and colloid chemistry. New York: CRC Press; 1997. p. 155–96.
Dalvi SV, Dave RN. Controlling particle size of a poorly water-soluble drug using ultrasound and stabilizers in antisolvent precipitation. Ind Eng Chem Res. 2009;48:7581–93.
Mansour HM, Sohn MJ, Al-Ghananeem A, DeLuca PP. Materials for pharmaceutical dosage forms: molecular pharmaceutics and controlled release. Int J Mol Sci. 2010;11:3298–322.
Devi DR, Sandhya P, Vedha Hari BNV. Poloxamer: a novel functional molecule for drug delivery and gene therapy. J Pharm Sci Res. 2013;5:159–65.
Yining L, Paschalis A. Temperature-dependent adsorption of pluronic F127 block copolymers onto carbon black particles dispersed in aqueous media. J Phys Chem. 2002;106:10834–44.
Nagarajan R. Solubilization of hydrocarbons and resulting aggregate shape transitions in aqueous solutions of Pluronic® (PEO–PPO–PEO) block copolymers. Colloids Surf B: Biointerfaces. 1999;16:55–72.
Mansour HM, Sohn M, Al-Ghananeem A, DeLuca PP. Materials for pharm. Aceutical dosage forms: molecular pharmaceutics and controlled release drug delivery. Int J Mol Sci. 2010;11:3298–322. doi:10.3390/ijms11093298.
Van Der Schaaf PA, Schwarzenbach F, Kirner H-J, Szelagiewicz M, Marcolli C, Burkhard A, et al. Polymorphic forms of sertraline hydrochloride, US 6872853 B1.
He Q, Rohani S, Zhu J, Gomaa H. Sertraline racemate and enantiomer: solid-state characterization, binary phase diagram, and crystal structures. Cryst Growth Des. 2010;10:1633–45.
Lu J. Crystallization and transformation of pharmaceutical solid forms. Afr J Pharm Pharmacol. 2012;6:581–891.
Censi R, Di Martino P. Polymorph impact on the bioavailability and stability of poorly soluble drugs. Molecules. 2015;20:18759–76. doi:10.3390/molecules201018759.
Schwartz E, Nidam T, Liberman A, Mendelovici M, Aronhime J, Singer C, et al. Methods for preparation of Sertraline hydrochloride polymorphs. US patent 6600073.
Patil S, Pawar A, Kumar S. Effect of additives on the physicochemical and drug release properties of pioglitazone hydrochloride spherical agglomerates. Trop J Pharm Res. 2012;11:18–27.
Abdullah EC, Geldart D. The use of bulk density measurements as flowability indicators. Powder Technol. 1999;102:151–65.
Shah KU, Khan GM. Regulating drug release behavior and kinetics from matrix tablets based on fine particle-sized ethyl cellulose ether derivatives: an in vitro and in vivo evaluation. Sci World J. 2012. doi:10.1100/2012/842348.
Tablet Friability. Pharmacopeial Forum: Volume No. 30(5) 32 page 1740. http://www.pharmacopeia.cn/v29240/usp29nf24s0_c1216.html. Accessed 2 June 2016.
Saleem M, Shahin M, Srinivas B, Begum A. Evaluation of tablets by friability apparatus. Int J Res Pharm Chem (IJRPC). 2014;4:837–40.
Santos OMM, Reis MED, Jacon JT, Lino ME, Simões JS, Doriguetto AC. Polymorphism: an evaluation of the potential risk to the quality of drug products from the Farmácia Popular Rede Própria. Braz J Pharm Sci. 2014. doi:10.1590/S1984-8250201100010000.
ACKNOWLEDGMENTS
The authors would like to acknowledge the faculty of scientific research at Jordan University of Science and Technology for financially supporting this research through grant number 53/2014.
Also, they would like to acknowledge Hikma Pharmaceuticals, Jordan, for donating sertraline hydrochloride, Al-Hayat Pharmaceutical Industries, Jordan, for donating venlafaxine hydrochloride and Evonik, Germany, for donating Eudragit® L100.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Al-Nimry, S.S., Jaber, M.A. Preparation and Optimization of Sertraline Hydrochloride Tablets with Improved Dissolution Through Crystal Modification. AAPS PharmSciTech 18, 1190–1202 (2017). https://doi.org/10.1208/s12249-016-0586-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-016-0586-z