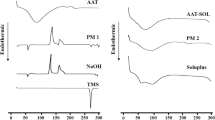
Abstract
Polymer-based amorphous solid dispersion (PASD) technology has attracted attention as one of the most feasible approaches for improving the solubility, dissolution rate, and bioavailability of insoluble drugs. Tegoprazan (TPZ) is a promising new drug used to treat gastroesophageal reflux disease with poor water solubility (∼0.03 mg/mL). This study developed novel PASD materials containing TPZ. Three polymers were used for this study: PVP, HPMCAS, and carbomer. The PASD powders were prepared via solvent evaporation at 50% drug loading. The physico-chemical properties of PASD solids were characterized using PXRD, MDSC, TGA, FT-IR, 1H SS-NMR, and stability testing. PASD powders fabricated with the neutral polymer PVP showed poor stability against drug crystallization. In contrast, those prepared using HPMCAS and carbomer showed no signs of crystallization even after three months of storage at 40oC/75% RH. A correlation between intermolecular interaction and physical stability was inferred for the TPZ PASD formulations. Amorphization of the crystalline TPZ with HPMCAS and carbomer resulted in a greatly increased in vitro dissolution rate. These two polymers showed similar performance, eliciting appreciable improvement in the in vivo absorption tests in rats. In summary, PASD formulations using acidic polymers (HPMCAS and carbomer) are novel formulations for improving the therapeutic effects of TPZ.
Similar content being viewed by others
Abbreviations
- ASD:
-
amorphous solid dispersions
- ATR:
-
attenuated total reflectance
- AUC:
-
area under curve
- BCS:
-
biopharmaceutical classification system
- CCDC:
-
cambridge crystallographic data center
- DSC:
-
differential scanning calorimetry
- FT-IR:
-
Fourier transform-infrared
- GERD:
-
gastroesophageal reflux disease
- HPMCAS:
-
hydroxypropyl methylcellulose acetate succinate
- MDSC:
-
modulated differential scanning calorimetry
- PAA:
-
polyacrylic acid
- PASD:
-
polymer-based amorphous solid dispersion
- PDF:
-
pair distribution function
- PPI:
-
proton pump inhibitors
- PVP:
-
polyvinylpirrolidone
- PXRD:
-
powder x-ray diffraction
- SS-NMR:
-
solid-state nuclear magnetic resonance
- TDA:
-
total diffraction analysis
- TGA:
-
thermogravimetric analysis
- Tg :
-
glass transition temperature
- T m-calc g :
-
calculated glass transition temperature of ASD
- T m-meas g :
-
measured glass transition temperature of ASD
- TPZ:
-
tegoprazan
References
W. L. Chiou and S. Riegelman, J. Pharm. Sci., 60, 1281 (1971).
S. V. Bhujbal, B. Mitra, U. Jain, Y. Gong, A. Agrawal, S. Karki, L. S. Taylor, S. Kumar and Q. Zhou, Acta Pharm. Sin. B., 11, 2505 (2021).
R. Iyer, V. P. Jovanovska, K. Berginc, M. Jaklič, F. Fabiani, C. Harlacher, T. Huzjak and M. V. Sanchez-Felix, Pharmaceutics, 13, 1682 (2021).
P. Pandi, R. Bulusu, N. Kommineni, W. Khan and M. Singh, Int. J. Pharm., 586, 119560 (2020).
D. M. Walden, Y. Bundey, A. Jagarapu, V. Antontsev, K. Chakravarty and J. Varshney, Molecules, 26, 182 (2021).
J. Liu, H. Grohganz, K. Löbmann, T. Rades and N. J. Hempel, Pharmaceutics, 13, 389 (2021).
C. G. Bavnhøj, M. M. Knopp, C. M. Madsen and K. Löbmann, Int. J. Pharm. X, 1, 100008 (2019).
N. J. Hempel, K. Brede, N. E. Olesen, N. Genina, M. M. Knopp and K. Löbmann, Int. J. Pharm., 544, 153 (2018).
E. O. Kissi, M. T. Ruggiero, N. J. Hempel, Z. Song, H. Grohganz, T. Rades and K. Löbmann, Phys. Chem. Chem. Phys., 21, 19686 (2019).
H. Grohganz, P. A. Priemel, K. Löbmann, L. H. Nielsen, R. Laitinen, A. Mullertz, G. V. D. Mooter and T. Rades, Expert Opin. Drug Deliv., 11, 977 (2014).
N. K. Duggirala, J. Li, N. S. K. Kumar, T. Gopinath and R. A. Suryanarayanan, ChemComm., 55, 5551 (2019).
P. Mistry, S. Mohapatra, T. Gopinath, F. G. Vogt and R. Suryanarayanan, Mol. Pharm., 12, 3339 (2015).
H. Nie, Y. Su, M. Zhang, Y. Song, A. Leone, L. S. Taylor, P. J. Marsac, T. Li and S. R. Byrn, Mol. Pharm., 13, 3964 (2016).
S. Bandari, S. Jadav, B. B. Eedara, R. Jukanti and P. R. Veerareddy, Korean J. Chem. Eng., 30, 238 (2013).
K. Yuvaraja, S. K. Das and J. Khanam, Korean J. Chem. Eng., 32, 132 (2015).
H. Rostamian, M. N. Lotfollahi and A. Mohammadi, Korean J. Chem. Eng., 37, 2295 (2020).
K. Kothari, V. Ragoonanan and R. Suryanarayanan, Mol. Pharm., 12, 162 (2015).
T. Miyazaki, S. Yoshioka, Y. Aso and S. Kojima, J. Pharm. Sci., 93, 2710 (2004).
A. C. Rumondor and L. S. Taylor, Mol. Pharm., 7, 477 (2010).
D. Yu, J. Li, H. Wang, H. Pan, T. Li, T. Bu, W. Zhou and X. Zhang, Eur. J. Pharm. Sci., 169, 106086 (2022).
N. G. Solanki, K. Lam, M. Tahsin, S. G. Gumaste, A. V. Shah and A. T. M. Serajuddin, J. Pharm. Sci., 108, 1453 (2019).
S. Wang, C. Liu, Y. Chen, A. Zhu and F. Qian, Mol. Pharm., 15, 4643 (2018).
X. Yao, A. L. Neusaenger and L. Yu, Pharmaceutics, 13, 1271 (2021).
X. Liu, X. Feng, R. O. Williams and F. Zhang, J. Pharm. Investig., 48, 19 (2018).
S. A. Raina, D. E. Alonzo, G. G. Z. Zhang, Y. Gao and L. S. Taylor, Mol. Pharm., 11, 3565 (2014).
Y. Song, X. Yang, X. Chen, H. Nie, S. Byrn and J. W. Lubach, Mol. Pharm., 12, 857 (2015).
M. S’ari, H. Blade, S. Cosgrove, R. Drummond-Brydson, N. Hondow, L. P. Hughes and A. Brown, Mol. Pharm., 18, 1905 (2021).
H. Mori and H. Suzuki, J. Neurogastroenterol. Motil., 25, 6 (2019).
S. Han, H. Y. Choi, Y. H. Kim, J. Y. Nam, B. Kim, G. S. Song, H. S. Lim and K. S. Bae, Aliment Pharmacol. Ther., 50, 751 (2019).
N. Takahashi and Y. Take, J. Pharmacol. Exp. Ther., 364, 275 (2018).
J. G. Hwang, H. Yoo, J. W. Lee, G. S. Song, S. Lee and M. G. Kim, Transl. Clin. Pharmacol., 27, 80 (2019).
J. Ghim, M. C. Chin, J. Jung, J. Lee, S. Kim, B. Kim, G. S. Song, Y.-K. Choi and J.-G. Shin, J. Clin. Pharmacol., 61, 913 (2020).
Y. J. Kim, E. S. Kim, J. Y. Lee, H. W. Lee, J. H. Kweon, S. A. Lee, K. D. Choi, D. H. Ko and S. P. Heo, Korea Patent, KR101684053 B1 (2016).
E. S. Kim, M. K. Lee, S. A. Lee, K. D. Choi, J. S. Kim and H. C. Yoo, Korea Patent, KR101829706 B1 (2018).
D. T. Friensen, R. Shanker, M. Crew, D. T. Smithey, W. J. Curatolo and J. A. S. Nightingale, Mol. Pharm., 5, 1003 (2008).
M. Gordon and J. S. Taylor, J. Appl. Chem., 2, 493 (1952).
B. C. Hancock, P. York and R. C. Rowe, Int. J. Pharm., 148, 1 (1997).
D. J. Greenhalgh, A. C. Williams, P. Timmins and P. York, J. Pharm. Sci., 88, 1182 (1999).
L. Glasser, J. Chem. Educ., 88, 581 (2011).
S. Jankovic, G. Tsakiridou, F. Ditzinger, N. J. Koehl, D. J. Price, A. R. Ilie, L. Kalantzi, K. Kimpe, R. Holm, A. Nair, B. Griffin, C. Saal and M. Kuentz, J. Pharm. Pharmacol., 71, 441 (2019).
T. Nakano, N. Saito and H. Minami, Langmuir, 36, 11957 (2020).
L. Li, Z. Jiang, J. Xu and T. Fang, J. Appl. Polym. Sci., 131, 40304 (2014).
F. Qian, J. Huang and M. A. Hussain, J. Pharm. Sci., 99, 2941 (2010).
K. Lehmkemper, S. O. Kyeremateng, O. Heinzerling, M. Degenhardt and G. Sadowski, Mol. Pharm., 14, 4374 (2017).
Lubrizol limited. Material safety data sheet (2018). https://www.lubrizol.com. Accessed 13 Apr 2022.
F. Meng, A. Trivino, D. Prasad and H. Chauhan, Eur. J. Pharm. Sci., 71, 12 (2015).
F. Qian, J. Huang, Q. Zhu, R. Haddadin, J. Gawel, R. Garmise and M. Hussain, Int. J. Pharm., 395, 232 (2010).
S. B. Teja, S. P. Patil, G. Shete, S. Patel and A. K. Bansal, J. Excip. Food Chem., 4, 1048 (2016).
T. T. Tran and P. H. Tran, Pharmaceutics, 12, 745 (2020).
B. V. Eerdenbrugh and L. S. Taylor, CrystEngComm., 13, 6171 (2011).
T. Fornaro, D. Burini, M. Biczysko and V. Barone, J. Phys. Chem. A, 119, 4224 (2015).
F. M. Iqbal, M. Ahmad and U. R. Tulain, Acta Poloniae Pharm., 74, 527 (2017).
X. Yuan, T. X. Xiang, B. D. Anderson and E. J. Munson, Mol. Pharm., 12, 4518 (2015).
S. Mohan, N. Sundaraganesan and J. Mink, Spectrochim. Acta A, 47, 1111 (1991).
Z. Shang, L. Yang and G. Chang, Polym. Int., 65, 332 (2016).
N. Vijayan, R. R. Babu, R. Gopalakrishnan, P. Ramasamy and W. T. A. Harrison, J. Cryst. Growth, 262, 490 (2004).
Y. Ishizuka, K. Ueda, H. Okada, J. Takeda, M. Karashima, K. Yazawa, H. Higashi, K. Kawakami, Y. Ikeda and K. Moribe, Mol. Pharm., 16, 2785 (2019).
L. A. Wegiel, L. J. Mauer, K. J. Edgar and L. S. Taylor, J. Pharm. Sci., 102, 171 (2013).
J. Dong, Y. Ozaki and K. Nakashima, Macromolecules, 30, 1111 (1997).
H. Konno and L. S. Taylor, J. Pharm. Sci., 95, 2692 (2006).
Merck. IR Spectrum Table & Chart. https://www.sigmaaldrich.com/KR/ko/technical-documents/technical-article/analytical-chemistry/photometry-and-reflectometry/ir-spectrum-table. Accessed 24 Jan 2022.
U. Eduok, O. Faye and J. Szpunar, RSC Adv., 6, 108777 (2016).
J. A. Marks, L. A. Wegiel, L. S. Taylor and K. J. Edgar, J. Pharm. Sci., 103, 2871 (2014).
H. Honda, Molecules, 18, 4786 (2013).
A. S. Tatton, T. N. Pham, F. G. Vogt, D. Luga, A. J. Edwards and S. P. Brown, Mol. Pharm., 10, 999 (2013).
Ü. Akbey, R. Graf, Y. G. Peng, P. P. Chu and H. W. Spiess, J. Polym. Sci. B Polym. Phys., 47, 138 (2009).
S. Ando, J. Kikuchi, Y. Fujimura, Y. Ida, K. Higashi, K. Moribe and K. Yamamoto, J. Pharm. Sci., 101, 3214 (2012).
F. Grifasi, M. R. Chierotti, K. Gaglioti, R. Gobetto, L. Maini, D. Braga, E. Dichiarante and M. Curzi, Cryst. Growth Des., 15, 1939 (2015).
E. Browne, Z. A. Worku and A. M. Healy, Pharmaceutics, 12, 433 (2020).
D. S. Frank and A. J. Matzger, Mol. Pharm., 15, 2714 (2018).
Q. He, J. Liu, J. Liang, X. Liu, D. Tuo and W. Li, Materials, 11, 247 (2018).
M. Monschke and K. G. Wagner, Pharmaceutics, 12, 541 (2020).
N. G. Solanki, K. Lam, M. Tahsin, S. G. Gumaste, A. V. Shah and A. T. M. Serajuddin, J. Pharm. Sci., 108, 1453 (2019).
T. Swift, L. Swanson, M. Geoghegan and S. Rimmer, Soft Matter., 12, 2542 (2016).
V. Wilson, X. Lou, D. J. Osterling, D. F. Stolarik, G. Jenkins, W. Gao, G. G. Z. Zhang and L. S. Taylor, J. Control Rel., 292, 172 (2018).
M. O. Jara, Z. N. Warnken and R. O. Willams III, Pharmaceutics, 13, 97 (2021).
E. L. McConnell, A. W. Basit and S. Murdan, J. Pharm. Pharmacol., 60, 63 (2008).
Acknowledgements
This research was financially supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF-2020R1F1A106966813). This study was also supported by the Soonchunhyang University Research Fund.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supporting Information
Additional information as noted in the text. This information is available via the Internet at http://www.springer.com/chemistry/journal/11814.
Electronic Supplementary Material
11814_2022_1280_MOESM1_ESM.pdf
Amorphous solid dispersions of tegoprazan and three different polymers: In vitro/in vivo evaluation of physicochemical properties
Rights and permissions
About this article
Cite this article
Kim, P., Lee, IS., Kim, JY. et al. Amorphous solid dispersions of tegoprazan and three different polymers: In vitro/in vivo evaluation of physicochemical properties. Korean J. Chem. Eng. 40, 986–998 (2023). https://doi.org/10.1007/s11814-022-1280-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-022-1280-3