Abstract
Polyvinyl alcohol (PVAL) has not been investigated in a binary formulation as a concentration-enhancing polymer owing to its high melting point/high viscosity and poor organic solubility. Due to the unique attributes of the KinetiSol® dispersing (KSD) technology, PVAL has been enabled for this application and it is the aim of this paper to investigate various grades for improvement of the solubility and bioavailability of poorly water soluble active pharmaceutical ingredients. Solid amorphous dispersions were created with the model drug, itraconazole (ITZ), at a selected drug loading of 20%. Polymer grades were chosen with variation in molecular weight and degree of hydroxylation to determine the effects on performance. Differential scanning calorimetry, powder X-ray diffraction, polarized light microscopy, size exclusion chromatography, and dissolution testing were used to characterize the amorphous dispersions. An in vivo pharmacokinetic study in rats was also conducted to compare the selected formulation to current market formulations of ITZ. The 4-88 grade of PVAL was determined to be effective at enhancing solubility and bioavailability of itraconazole.
Similar content being viewed by others
INTRODUCTION
Poor water solubility has been documented as a common characteristic for new chemical entities in development pipelines and commercially available products in the pharmaceutical industry today (1–3). Among the options for dealing with poor solubility, the incorporation of the drug substance in a solid amorphous dispersion dosage form is gaining popularity. There are many reasons for this increase in popularity with only a few being mentioned here. First, conventional chemistry methods for solubility enhancement like salt formation reportedly only work for 20–30% of new molecules, leaving the 70–80% remaining to find other routes to improved solubility (4). Second, solid dispersions containing solubility-enhancing polymers can dramatically increase apparent solubility for durations sufficient to enable absorption form the intestinal lumen. Specifically, in the case of ITZ, solubility increases of 10,000-fold or more have been demonstrated (5–7). Third, nano-crystal technologies have not proven as capable and industry trends show preference to solid dispersions (8). Fourth, compared with other non-crystalline drug delivery approaches such as cosolvent systems or self-emulsifying drug delivery systems (SEDDS), amorphous solid dispersions are more amenable to be developed into tablets, which are the preferred solid dosage form for distribution, and amorphous dispersions demonstrate highly desirable advantages over liquid or semisolid formulations including lower manufacturing cost, smaller pill burden, and improved physical and chemical stability (9). Finally, the various grades of polymers used for solid dispersions can not only increase solubility but also allow for targeted release and the tailoring of release profiles of a drug substance, thus solving multiple problems simultaneously.
While numerous studies have been published on amorphous solid dispersions, there are a limited number of polymers suitable for creating those systems. Polymers that have been reported as a major component in solid dispersion formulations for improved solubility are as follows: povidone (PVP) (10). copovidone (PVPVA) (11). crospovidone (CrosPVP) (12). polyethyleneglycols (PEG) (13,14). polymethacrylates (15,16). hydroxypropylmethylcellulose or hypromellose (HPMC) (17,18). hypromellose acetate succinate (HPMCAS) (19,20). hypromellose phthalate (HPMCP) (21). hydroxyethylcellulose (HEC) (22). hydroxypropylcellulose (HPC) (23). polyvinyl acetate phthalate (PVAP) (5). cellulose acetate phthalate (CAP) (5). poloxamers (24). carbomers (25). and Soluplus® (26). As additional polymers are identified as options, a broader range of challenging drug substances may be enabled for oral delivery systems. Furthermore, existing prohibitive intellectual property could be circumvented and unique release profiles could be created. Due to the high cost of development and regulatory compliance verification, repurposing an existing excipient would seem a financial and time efficient path.
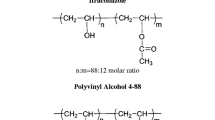
Polyvinyl alcohol (PVAL) is a water-soluble synthetic polymer represented by the formula (C2H4O) n . The value of n for commercially available materials is between 500 and 5000, which is roughly equivalent to a molecular weight range of 20,000 to 200,000 (27). PVAL is unique among the vinyl polymers in the fact that the monomer, vinyl alcohol, cannot exist in the free form. So, it is manufactured by the polymerization of vinyl acetate and then converted by a hydrolysis (alcoholysis) process. Various grades based on extent of hydrolysis exist with unconverted fractions being polyvinyl acetate. Since EMD Millipore’s PVAL was used in this research, a brief explanation of their nomenclature is prudent. Each grade of PVAL has two groups of numbers separated by a dash. The first group of numbers represents the viscosity of the 4% aqueous solution at 20°C as a relative indication of the molar mass. The second group of numbers is the degree of hydrolysis of the polyvinyl acetate. For example, an 88 in the second group would represent 88% hydrolysis with 98% being considered a fully hydrolyzed grade. Thus, a 5-88 grade would have the viscosity of 5 mPa·s in a 4% aqueous solution at 20°C with the polymer chain being approximately 88% PVAL and 12% polyvinyl acetate (28). Table I contains additional properties.
PVAL is currently being used in a variety of pharmaceutical applications. It is used as a stabilizing agent for emulsions (29). in topical pharmaceutical (30). and in ophthalmic formulations (31,32). It is used in artificial tears (33) and integrated into contact lenses for lubrication purposes (34). Polyvinyl alcohol can be made into microspheres (35) and is used as an emulsifier in creating PLGA nanoparticles (36,37). It has also been used in sustained-release formulations for oral administration (38) and transdermal patches (39). Oral toxicity has been evaluated and found safe even at high levels of consumption; the no-observed-adverse-effect level (NOAEL) for rats in a 3-month study was 5000 mg/kg body weight/day (40).
To the authors’ knowledge, PVAL has not been reported as the polymeric carrier in binary amorphous solid dispersions (41). A brief discussion of the limitations of conventional methods for creating amorphous dispersions provides insight to probable reasons for this. Solvent processes (e.g., like spray drying and precipitation approaches) require a drug/polymer solution prepared with a common solvent or cosolvents. PVAL is insoluble in most organic solvents and only slightly soluble in ethanol while typical pipeline drug molecules are only slightly soluble in more aggressive organic solvents. Even if suitable solvents were ascertained, the required concentration of polymer would render the solution too viscous for practical processing (like preventing flow through a small orifice needed for spray drying). Alternatively, the application of conventional fusion processes like hot melt extrusion (HME) and injection molding is limited due to the high melting point of PVAL and high viscosity of the resulting melt. These technologies are not suitable for processing thermally sensitive and viscous formulations; however, KinetiSol® has been demonstrated in these applications (42). KinetiSol® dispersing is a novel fusion process that does not have torque limitations like HME and has been shown to create unique solid dispersions (43). Through this enabling technology, solid amorphous dispersions containing PVAL as the primary polymer can now be evaluated.
The pH independence of PVAL presents some advantages in bioavailability enhancement. The dissolution of pharmaceutical excipients with pH-dependent ionizing properties can be variable across the gastrointestinal pH range (44). For example, dissolution rate and apparent solubility of weakly basic drugs in gastric pH and weakly acidic drugs in intestinal pH would be higher due to the ionization at those pH conditions (6,7,45). Anionic and cationic polymers are prone to those same ionization effects, which allow for differing dissolution rates across the gastrointestinal tract. This may become clinically significant for formulations utilizing ionic polymers in both inter-patient and intra-patient variation. The dissolution behavior of non-ionic polymers is expected to be the same across the gastrointestinal tract, which should lower variability and provide a more predictable bioavailability improvement (46). Anionic polymers, even with variation challenges, are still often chosen in formulation development due to their ability to improve solubility compared to non-ionic polymers (47). Anionic polymers have been shown to increase dissolution rate and apparent solubility of both weakly basic and neutral drugs in amorphous dispersions due to polymer/drug interactions likely occurring between polymer functional groups and the drug substance (48). The few non-ionic polymers used for solid dispersions, primarily HPMC, PVP, and PVPVA, do not exhibit tunable ratios of hydrogen bond donors and receptors like HMPCAS and polymethacrylates. However, PVAL has a tunable density of hydrogen bond donating hydroxyl groups and hydrogen bond receptors along its polymer backbone while still having pH-independent solubility. In theory, this would allow it to function strongly as a solubility enhancer like the anionic polymers but also have the low variability of the non-ionic polymers.
It is hypothesized that the pH-independent solubility along with tunable density of functional groups of PVAL would be ideal in the creation of solid dispersions especially for weakly basic drugs. While other groups have shown stability with semicrystalline polymers, PVAL will also need to be investigated to establish that the polymer crystalline structure does not create nucleation sites for drug recrystallization during processing and storage (49). As a first step in investigating that PVAL will function as a solubility-enhancing polymer, ITZ was selected as the model drug to be formulated in various grades. Varying molecular weight grades with identical degree of hydrolysis were used to create solid dispersions to determine its effect on performance. Conversely, the best performing molecular weight range of PVAL from the first group of formulations was selected in grades of changing degrees of hydrolysis. The best performing polymer grade would then be compared to commercially available ITZ products.
MATERIALS
Itraconazole was purchased from Hawkins, Inc. (Minneapolis, MN). All grades of PVAL (EMD Millipore) were donated by Merck Millipore. All other chemicals used in this study were of ACS grade.
METHODS
KinetiSol® Dispersing
All compositions for this study were produced by a lab-scale, GMP pharmaceutical compounder designed and manufactured by DisperSol Technologies, L.L.C. (Georgetown, TX, USA). Prior to KinetiSol® dispersing (KSD) processing, materials were weighed, placed into a polyethylene bag, manually shaken for approximately 1 min, and charged into the compounder chamber. Ninety-gram batches were typically processed at 2800 rpm and ejected at 170°C with slight parameter adjustments made for higher viscosity grades. During processing, real-time computer controls monitor various parameters and eject the material at a pre-set ejection temperature. Discharged material was immediately quenched in a cooling die under pressure in a pneumatic press. Cooled material was then milled in a FitzMill L1A and screened through a 60-mesh screen (250 μm). All further analyses and tests were conducted on this powder.
Differential Scanning Calorimetry
Modulated differential scanning calorimetry (DSC) analysis was conducted using a TA Instruments Model mQ20 DSC (New Castle, DE) equipped with a refrigerated cooling system and autosampler. Samples were weighed to 5–10 mg in aluminum-crimped pans. Samples were heated at a ramp rate of 10°C/min from 0 to 225°C with a modulation temperature amplitude of 1.0°C and a modulation period of 60 s for all studies. Ultrahigh purity nitrogen was used as the purge gas at a flow rate of 50 mL/min. All data analyses were performed using TA Universal Analysis software. The thermogram for amorphous ITZ used in the DSC analysis of the solid dispersions formulations was obtained on a second heating of crystalline ITZ following an initial heating to 200°C followed by rapid cooling (40°C/min) to 0°C. Multiple runs were conducted with representative data selected for illustrations.
Powder X-Ray Diffraction
An Inel Equinox 100 X-ray diffractometer (INEL, Artenay, France) was used to detect the presence of ITZ crystallinity. Milled compositions, physical mixtures, or unprocessed ITZ were loaded on a rotating aluminum sample holder and placed in the radiation chamber. The Equinox 100 utilizes Cu K Alpha radiation (λ = 1.5418 Å) with a curved radius detector to simultaneously measure a 2ϴ range of 5–110°. Operating voltage and amperage were adjusted to 41 kV and 0.8 mA, respectively, and the scan time for each sample was 10 min.
Polarized Light Microscopy
While powder X-ray diffraction (XRD) has been shown to have limits of detection of less than 1% (50). polarized light microscopy (PLM) can serve as a qualitative confirmation of XRD results. PLM analysis was conducted on a Meiji Techno MT 9300 polarizing light microscope with a first-order red compensator. Pulverized samples were dusted on a glass slide and viewed under ×400 magnification. The slide holder was rotated at least 90° while being observed to detect any light refractions. Images were taken by an Infinity CMOS camera.
Size Exclusion Chromatography
Molecular weights of PVAL in both processed and unprocessed samples were analyzed with a Dionex Ultimate 3000 H/UPLC system equipped with a Shodex (Showa Denko K.K., Japan) Refractive Index detector. Two Shodex Asahipak 7.6 mm × 300 mm (GF-7 M HQ) columns were placed in series and maintained at 40°C. A 50-nM LiCl aqueous solution was utilized as the mobile phase at a constant flow rate of 0.7 mL/min. Samples were prepared at a concentration of 0.5 g/L in mobile phase, and injection volumes were 100 μL (51).
Non-sink Dissolution Analysis
Non-sink dissolution analysis was conducted with a VK 7000 dissolution tester (Varian, Inc., Palo Alto, CA, USA) in accordance with USP XXXIV Method A for delayed release dosage forms. Solid dispersions (n = 3) were weighed to achieve an equivalent of 37.5 mg ITZ and placed in dissolution vessels containing 750 mL of 0.1 N HCl (∼10× ITZ equilibrium solubility in acid) equilibrated to a temperature of 37.0 ± 1°C with a paddle rotation of 50 rpm. After 2 h, a buffer medium of 250 mL of 0.2 M Na3PO4, preheated to 37.0 ± 1°C, was added to the dissolution vessels to adjust the pH to 6.80 and reach 1000 mL in volume. Samples were taken at 1, 2, 2.25, 2.5, 3, 4, and 5 h. Samples were immediately filtered using 0.2-μm PTFE membrane 13-mm filters, diluted to a 1:1 ratio with mobile phase, mixed, and then transferred into 1-mL vials for HPLC analysis.
High-Performance Liquid Chromatography
The ITZ content in processed samples and dissolution aliquots was analyzed with a Dionex Ultimate 3000 H/UPLC system equipped with diode array detector extracting at 263 nm. The system was operated under isocratic conditions with a 70:30:0.05 acetonitrile/water/diethanolamine mobile phase equipped with a Phenomenex Luna 5 μm C18(2) 100 Å, 150 mm × 4.6 mm (Phenomenex, Torrance, CA) HPLC column. Dionex Chromeleon 7.2 software was used to analyze all chromatography data.
In Vivo Studies
In vivo studies were conducted at Charles River Laboratories (Wilmington, Massachusetts Facility) under NIH guidelines with IACUC approval. Male Sprague–Dawley rats equipped with a surgically implanted jugular vein catheters to facilitate blood collection were prepared for the study. Animals were assigned to the study based on catheter patency and acceptable health as determined by a staff veterinarian and placed into groups of four animals (See Table II for details). All animals were fasted overnight prior to dose administration, and food was returned following the 4-h post-dose blood collection. All dosage forms were milled to a fine powder and suspended in an aqueous dosing vehicle to insure accurate ITZ to rat body weight medicating.
Blood samples (0.25 mL; sodium heparin anti-coagulant) were collected from the jugular vein catheter or by venipuncture of a tail vein if the catheter became blocked. Blood samples were collected from each animal at 2, 3, 3.5, 4, 5, 6, 8, 12, and 24 h following oral dosing. All whole blood samples were placed on wet ice immediately after collection and were centrifuged at 2–8°C to isolate plasma. The resulting plasma was transferred to individual polypropylene tubes and immediately placed on dry ice until storage at nominally −20°C before transfer to the Charles River’s Bioanalytical Chemistry Department for concentration analysis.
Plasma samples were analyzed for itraconazole concentration using a proprietary in-house research grade LC-MS/MS Assay. Pharmacokinetic parameters were estimated from the plasma concentration–time data using standard non-compartmental methods and utilizing suitable analysis software (Watson 7.2 Bioanalytical LIMS, Thermo Electron Corp).
RESULTS AND DISCUSSION
Preliminary Evaluation of PVAL Molecular Weight
Due to the uniqueness of the KinetiSol® technology, screening methods do not exist to determine the processability of a new polymer. So, three viscosity grades representing a low (PVAL 4-88), medium (PVAL 26-88), and high (PVAL 40-88) with the same level of hydrolysis were selected with an arbitrary ITZ loading of 20%. Because of the feasibility nature of this study, KSD processing parameters were not optimized but considered acceptable if ITZ was rendered amorphous as determined by XRD and PLM analysis and drug assays were at least 96%. This was easily achieved for the 4-88 grade as the discharged material was a single agglomerated, semi-molten mass similar in character to other previously processed formulations. The 26-88 and 40-88 grades behaved differently in that the discharged material was a slightly densified powder. Variance in processing RPM or increases in discharge temperature would not render an agglomerated discharge which is typical of the KSD processes (preliminary results not reported). Other polymers with larger molecular weights have been used in previous research without incident (52). Thus, this phenomena must be attributed to the specific sintering properties or crystalline nature of high viscosity grades of PVAL. The addition of 4-88 to 26-88 as a minor component allowed for an agglomerated discharge; however, a 1:1 ratio of 4-88 to 40-88 was required to achieve the same result. For consistency, a 1:1 ratio of 4-88 to both 26-88 and 40-88 was chosen as the compositions to continue this portion of the study.
Size exclusion chromatography was performed on unprocessed polymer grades as controls and on the KSD processed compositions of ITZ with 4-88, 4-88:26-88, and 4-88:40-88. Comparison of unprocessed and KSD processed 4-88 is shown in Fig. 1 while comparison of unprocessed, 1:1 physical mixture of 4-88 and 40-88 to the KSD process composition is shown in Fig. 2. Peaks associated with polymer have been numbered, followed by the elution time (longer elution times signifies a smaller molecular weight). Each sample was analyzed in triplicate, and representative chromatograms are presented herein. Unprocessed 4-88 had a peak elution time of 24.917 min compared to the KSD processed 4-88 with a peak time of 24.638. The physical mixture of 4-88 and 40-88 exhibited a first polymer peak at 21.193 min, which corresponds with the 40-88 grade, and the second peak at 24.788, which is associated with the 4-88. The KSD composition showed elution times of the respective peaks at 21.103 and 24.407. The small difference in the times can be explained by sampling variance, and it can be concluded that KSD processing did not change the molecular weight of PVAL. However, it was noted that the KSD compositions always eluded at an earlier time compared to the corresponding unprocessed powder, which would indicate a slight increase in molecular weight. It is plausible that a portion of the solubilized ITZ remains bound on the polymer following dissolution of the sample in the diluent and while traversing the column, which would increase the apparent size of the polymer coil and manifest as an increase in molecular weight. The key result of this analysis is that there is no indication of a reduction in the molecular weight of the starting PVAL material after KSD processing.
Dissolution profiles of the three compositions were compared (data not shown). The ITZ:4-88 and ITZ:4-88:26-88 compositions performed similarly, but the ITZ:4-88:40-88 composition did not maintain ITZ supersaturation to the same extent following the pH change. With its ease of processing and good dissolution profile, the 4 mPa·s grade of PVAL was selected as the molecular weight to continue evaluation of the effect of degree of hydrolysis on polymer performance.
Solid-State Analysis
Various hydrolysis grades of the same viscosity were selected for comparison with 4-88, namely, 4-38, 4-75, and 4-98. Polyvinyl alcohol is known to be a crystalline polymer, and XRD profiles were generated for each polymer used in this study to determine the effect of molecular weight and degree of hydrolysis on the crystallinity (see Fig. 3). The XRD data matches the reported information in that crystallinity does appear to increase with degree of hydrolysis. The 4-38 grade, which contains a greater component of polyvinyl acetate than PVAL, is the only grade that is XRD amorphous. All the other grades had a primary peak between 19 and 20 2-theta with two other easily distinguishable peaks between 22 and 23 and between 40 and 42 2-theta. The 26-88 and 40-88 grades showed larger peak intensities than the 4-88 grade that might indicate increased crystallinity with molecular weight and would explain why these grades were more difficult to process at the selected conditions. The 4-98 grade, which is the only fully hydrolyzed grade, demonstrated the highest peak intensities of all the grades tested.
XRD profiles were also generated for ITZ and the processed compositions as illustrated in Fig. 4. For the 4-38, 4-75, 4-88, and 4-98 compositions, the profile demonstrates that the drug substance is amorphous while the polymers retain their crystalline structure. In the compositions with a 1:1 polymer ratio of low molecular weight with higher molecular weight (only the 4-88:40-88 profile is shown, but the 4-88:26-88 profile was identical), the major crystalline peak is visible, but with lower intensity. Other peaks are barely distinguishable. It appears that the combination of different molecular weights interacts in such a way as to reduce the crystalline structure of the polymer portion of the composite.
Modulated differential scanning calorimetry (mDSC) analysis was conducted on the pure PVAL polymers to understand the thermal characteristic of each grade prior to analyzing the ITZ/PVAL KSD compositions. Considering that PVAL is a copolymer of polyvinyl acetate (PVAC) and PVAL, one would expect to see by DSC analysis a T g of the PVAC component and a melting endotherm of the crystalline PVAL component, the prominence of both being a function of the degree of hydrolysis. That is to say that with increasing degree of hydrolysis, the T g of the PVAC component will be less pronounced and the melting endotherm of PVAL will be more distinct. Thus, a fully hydrolyzed grade would exhibit essentially no T g associated with PVAC and a strong melting endotherm associated with PVAL. The DSC thermograms for the four 4 mPa·s grades of PVAL and their corresponding KSD compositions provided in Fig. 5 (non-reversing heat flow) and Fig. 6 (reversing heat flow) demonstrate this expected trend.
It is seen that the 4-38 grade, which is predominantly PVAC, exhibits a strong T g associated with the PVAC component and no detectable melting endotherm for PVAL (53). The amorphous nature of this grade as demonstrated by DSC is in agreement with the previously discussed XRD result. Hence, it is understood that below a critical degree of hydrolysis, the PVAL component is unable to interact with itself to form crystallite structures due to the density of PVAC on the polymer chain. Examining the thermograms for the 4-75 and the 4-88 grades, the expected trend is seen in that the T g becomes less noticeable and the melting endotherm becomes more pronounced with an increased T m as the degree of hydrolysis is increased: consistent with increasing crystalline content of the polymer. The thermal profile obtained for the 4-98 grade matches industry literature for fully hydrolyzed polyvinyl alcohol with a melting point around 223°C. The reversing heat flow profile for PVAL 4-98 shows a slight thermal event around 70°C which others have suggested was the T g (54). This event was much less prominent than the T g events for 4-88, 4-75, and 4-38 as would be expected considering the low PVAC content and was not able to be accurately quantified by the analysis software. The DSC profile for the 4-98 grade corroborates the XRD result indicating high crystallinity of the fully hydrolyzed grade.
mDSC analysis was performed on the ITZ/PVAL KSD compositions to investigate the dispersed state of ITZ in each polymer grade and to evaluate the influence of the dispersed drug on the thermal properties of the polymers. The non-reversing heat flow thermogram in Fig. 7 shows a general trend of broadening and/or reduction in the magnitude of the glass transition event for the 4-38, 4-75, and 4-88 grades relative to the pure polymers. This could be attributed to dilution and/or anti-plasticizing effect of the dispersed ITZ phase. Consistent with the pure polymer, a T g is not apparent for the KSD composition with the 4-98 grade. Also seen for each of the 4-38, 4-75, and 4-88 grades is an exothermic event indicative of ITZ crystallization with peak temperatures in the range of 117–127°C. Interestingly, the magnitude of the exothermic event is inversely proportional to the degree of hydrolysis. This suggests that the physical stability of an ITZ amorphous dispersion in PVAL increases with increasing degree of hydrolysis. One possible explanation for this is that increasing the degree of hydrolysis increases the density of hydrogen bond donor sites on the polymer backbone that could interact with the hydrogen bond acceptor sites on ITZ to form a stable complex (55). Another possible explanation is that the amphiphilic character of the polymer that stems from the ratio of hydrophilic PVAL-to-hydrophobic PVAC units approaches an optimum with regard to stabilizing interactions with ITZ as the percent hydrolysis is increased up to 88%.
When the degree of hydrolysis is low, as with the 4-38 grade, the dispersed ITZ phase would interact largely with the PVAC component which contains only hydrogen bond acceptor sites (assuming hydrogen bonding is forming the complex). Therefore, in this phase, the amorphous dispersion is stabilized by weaker intermolecular forces like Van der Waals forces. If the stable complex is formed by hydrophilic/hydrophobic microenvironments, then the fewer hydroxyl groups of the 4-38 grade would provide fewer areas for interaction. In either case, these weaker interactions are more easily disrupted by thermal perturbations; hence, post-T g phase separation of amorphous ITZ and subsequent crystallization during the temperature ramp are observed to a greater extent with reduced degree of hydrolysis (56). This is corroborated by the result for the fully hydrolyzed grade in which a crystallization exotherm was not detected.
Figure 7 also shows that for all KSD processed compositions, a minor ITZ endotherm was detected. For the KSD compositions with PVAL 4-38, 4-75, and 4-88, this endotherm is similar in magnitude to the crystallization exotherm, which suggest that the ITZ melting event is related entirely to ITZ that crystallized during the DSC scan. This result validates the findings of XRD in which no crystalline ITZ was detected. In the case of the 4-98 grade, where no crystallization exotherm was detected, the ITZ melting endotherm is likely due to residual crystalline ITZ remaining after processing. This could be due to incomplete dispersion of ITZ in the polymer on processing resulting from the highly crystalline nature and/or the high T m of the PVAL 4-98 grade. It could also be the result of amorphous ITZ being forced out of its interaction with the polymer as the highly crystalline grade returned to its preferred crystalline orientation on quenching. This result appears to contradict the XRD analysis; however, considering its small magnitude, the melting endotherm represents a small fraction of the dispersed ITZ, the crystalline peaks of which could be easily masked by the high-intensity peaks of PVAL 4-98.
Finally, it is interesting to note in Fig. 7 that the melting endotherms for the PVAL 4-75 and 4-88 are significantly reduced in comparison to the pure polymer, beyond what would be expected for a 20% dilution on the addition of ITZ. This suggests that the dispersed ITZ disrupts crystallization of PVAL upon cooling due to drug/polymer interactions. A similar melting endotherm, in terms of magnitude, between the pure polymer and the KSD composition for the 4-98 grade seems to suggest incomplete disruption of polymer crystallinity on processing and/or displacement of ITZ during the quenching process. Considering this, it seems that a sufficient fraction of PVAC on the polymer is required to disrupt PVAL crystallite structure to facilitate a molten transition by KSD processing and achieve a homogenous dispersion of the drug substance (57). Additionally, PVAC units interspersed on the polymer backbone may act as defects within the PVAL crystallite structure that effectively make available sites for ITZ/polymer interactions (14). However, one would expect to see a point of diminishing returns for increasing PVAC concentration as this would also lead to weaker drug/polymer interactions. From Fig. 7, it would appear that this optimum PVAL/PVAC ratio for ITZ is near that of the 4-88 grade.
The reversing heat flow shown in Fig. 8 shows similar trends to those of the non-reversing heat flow analysis, however with better resolution of the glass transition events. Specifically, the T gs of the KSD compositions with the 4-88 and 4-98 grades are able to be resolved by reversing heat flow analysis and have respective midpoint values of 58.05 and 51.78°C. Also provided in Fig. 8 is a thermogram for pure amorphous ITZ which shows a T g of 59°C followed by two endothermic events at 74 and 90°C that have been identified as mesophases of glassy itraconazole (58). In comparing all of the KSD compositions in the figure, the 4-88 grade has the highest composite T g. The T g is more than 10°C higher (roughly 20%) than KSD compositions with 4-38 and 4-75 grades as well as the pure 4-88 polymer and is close to the T g of pure amorphous itraconazole. The anti-plasticizing effect of amorphous ITZ on the 4-88 grade indicates strong positive drug–polymer interactions (16). The increased T g relative to the other PVAL grades seems to also support the previously discussed hypothesis regarding an optimal ratio of PVAC to PVAL at which hydrogen bonding or hydrophilic/hydrophobic locations are maximized. From these results, it can be concluded that 4-88 is the best performer of the PVAL grades tested from a thermodynamic standpoint.
Non-sink, Gastric Transfer Dissolution Analysis
As discussed previously, PVAL is an interesting new carrier polymer in the area of supersaturating amorphous solid dispersions owing to its non-ionic nature and the high density of hydrogen bond donor sites on the polymer chain. These attributes make it a particularly attractive concentration enhancing carrier for weakly basic drugs which have been shown to interact strongly with anionic carrier polymers to yield substantial improvements in apparent solubility (6). Especially in the case of the weakly basic drug ITZ, where the neutral solubility is orders of magnitude lower than in the acidic gastric environment. To investigate the concentration-enhancing effects of PVAL on ITZ, the dissolution properties of the four KSD compositions were evaluated by a non-sink, gastric transfer dissolution method. The results of this analysis are presented in Figs. 9 and 10.
Both the 4-75 and 4-88 compositions achieved complete drug dissolution before the pH change at 2 h, while the 4-38 and 4-98 grades did not. Polyvinyl acetate is not water soluble, which would explain why 4-38 did not go into solution like the higher hydrolyzed grades. Owing to its highly crystalline nature, the 4-98 grade dissolves at a very slow rate under the dissolution conditions of this study; hence, ITZ release from this composition is slow and incomplete. After the pH change, the 4-88 grade provided superior concentration-enhancing effect presumably due to the greater PVAL content on the polymer and increased probability of ITZ interaction with the polymer in solution. The 4-38 and the 4-98 grades provided essentially no concentration-enhancing effect due to the low concentration of dissolved polymer in the dissolution medium. Contrasting the dissolution areas-under-the-curve (AUCs) after the pH change gives a rank order of 4-88 > 4-75 > 4-98 > 4-38 with respective values of 640 ± 89, 442 ± 23, 130 ± 9, and 115 ± 8 mg·min. By way of comparison, DiNunzio et al. reported a post-pH change dissolution AUC for Sporanox® capsules of 226 ± 23 mg·min (5). In Fig. 10, these dissolution results are presented in terms of concentration at each time point (C) relative to the saturation concentration (C S) of ITZ at the respective pH. It should be noted that the C S for ITZ decreases dramatically from 4 μg/mL to 1 ng/mL when the pH of the media is changed from 1.2 to 6.8 (5). When plotted in this manner, the extent of supersaturation enabled by PVAL 4-88 at neutral pH becomes quite evident with solution concentrations nearly 9000 times the saturation concentration of ITZ. These results therefore demonstrate that PVAL is an effective concentration-enhancing polymer for ITZ and provides stabilization/prolongation of aqueous supersaturation.
In Vivo Study
From solid state and dissolution evaluation of PVAL grades with varying degrees of hydrolysis, PVAL 4-88 emerged as the top performing polymer. In order to investigate the effect of this polymer on the oral absorption of ITZ from an amorphous dispersion, the pharmacokinetics (PK) following oral administration to a rat model of this binary composition were comparatively evaluated against the commercially available ITZ product, Onmel™. The plasma concentration versus time profiles generated from this study are shown in Fig. 11 while the key pharmacokinetic parameters as calculated by non-compartmental analysis are provided in Table III with the addition of previously obtained results for Sporanox®. The dosing conditions for the previously obtained Sporanox® were different than those in the current study and, thus, listed only for reference.
The mean AUC and C max for the PVAL 4-88 composition were greater than those of Onmel™. However, it is noted that the drug loading of the ITZ/HPMC dispersion in the Onmel™ tablet is double that of the PVAL 4-88 composition. Similarly, the non-pareil beads in the Sporanox® composition are coated with a 40% w/w itraconazole and 60% w/w HPMC solid dispersion (59). Therefore, the contrast with commercial products is not a true apple-to-apple comparison. Further studies will evaluate PVAL compositions at 40% w/w loading to have a more comparable result. Yet, considering the exploratory nature of this study, the analogous in vivo performance of this first-generation PVAL composition to commercial products is noteworthy.
Future Studies
Future research with ITZ will evaluate the effect of molecular weight in the range between the 4-88 and 26-88 PVAL grades tested in this study on dissolution performance. It will also explore increased drug loading levels, and evaluate storage stability. Additional separate studies are planned to determine the broad application of PVAL evaluating amorphous dispersions with neutral and weakly acidic drug substances. This supplementary information would offer a foundation for development scientists, who are looking for new options in formulating challenging drug substances, to have confidence in exploring polyvinyl alcohol.
CONCLUSION
This feasibility study has demonstrated that PVAL’s pH-independent solubility along with tunable density of functional groups allows it to function as a concentration-enhancing polymer, effectively increasing the apparent solubility of poorly water soluble drug, itraconazole. The KinetiSol® technology enables the use of PVAL as the primary carrier for amorphous solid dispersion compositions. Solid-state and non-sink dissolution analysis revealed that the 88% hydrolyzed grade of PVAL was optimal for the ITZ compositions. Pharmacokinetic analysis in a rat model demonstrated that the ITZ/PVAL 4-88 composition yielded exposures that were comparable to Sporanox® and Onmel™.
References
Dahan A, Miller JM, Amidon GL. Prediction of solubility and permeability class membership: provisional BCS classification of the world’s top oral drugs. AAPS J. 2009;11(4):740–6.
Ku MS, Dulin W. A biopharmaceutical classification-based right-first-time formulation approach to reduce human pharmacokinetic variability and project cycle time from first-in-human to clinical proof-of-concept. Pharm Dev Technol. 2012;17(3):285–302.
Takagi T, Ramachandran C, Bermejo M, Yamashita S, Lawrence XY, Amidon GL. A provisional biopharmaceutical classification of the top 200 oral drug products in the United States, Great Britain, Spain, and Japan. Mol Pharm. 2006;3(6):631–43.
Serajuddin ATM, Pudipeddi M. Salt-selection strategies. Handbook of pharmaceutical salts: properties, selection, and use Weinheim: Wiley-VCH. 2008:135–60.
DiNunzio JC, Miller DA, Yang W, McGinity JW, Williams III RO. Amorphous compositions using concentration enhancing polymers for improved bioavailability of itraconazole. Mol Pharm. 2008;5(6):968–80.
Miller DA, DiNunzio JC, Yang W, McGinity JW, Williams RO. Targeted intestinal delivery of supersaturated itraconazole for improved oral absorption. Pharm Res. 2008;25(6):1450–9.
Miller DA, DiNunzio JC, Yang W, McGinity JW, Williams III RO. Enhanced in vivo absorption of itraconazole via stabilization of supersaturation following acidic-to-neutral pH transition. Drug Devel Ind Pharm. 2008;34(8):890–902.
Brough C, Williams Iii RO. Amorphous solid dispersions and nano-crystal technologies for poorly water-soluble drug delivery. Int J Pharm. 2013;453(1):157–66.
Qian F, Huang J, Hussain MA. Drug–polymer solubility and miscibility: stability consideration and practical challenges in amorphous solid dispersion development. J Pharm Sci. 2010;99(7):2941–7.
Van Drooge D, Hinrichs W, Visser M, Frijlink H. Characterization of the molecular distribution of drugs in glassy solid dispersions at the nano-meter scale, using differential scanning calorimetry and gravimetric water vapour sorption techniques. Int J Pharm. 2006;310(1):220–9.
Verreck G, Decorte A, Heymans K, Adriaensen J, Cleeren D, Jacobs A, et al. The effect of pressurized carbon dioxide as a temporary plasticizer and foaming agent on the hot stage extrusion process and extrudate properties of solid dispersions of itraconazole with PVP-VA 64. Eur J Pharm Sci. 2005;26(3):349–58.
Shibata Y, Fujii M, Kokudai M, Noda S, Okada H, Kondoh M, et al. Effect of characteristics of compounds on maintenance of an amorphous state in solid dispersion with crospovidone. J Pharm Sci. 2007;96(6):1537–47.
Urbanetz NA. Stabilization of solid dispersions of nimodipine and polyethylene glycol 2000. Eur J Pharm Sci. 2006;28(1):67–76.
Janssens S, de Armas HN, Remon JP, Van den Mooter G. The use of a new hydrophilic polymer, Kollicoat IR® in the formulation of solid dispersions of Itraconazole. Eur J Pharm Sci. 2007;30(3):288–94.
Albers J, Alles R, Matthée K, Knop K, Nahrup JS, Kleinebudde P. Mechanism of drug release from polymethacrylate-based extrudates and milled strands prepared by hot-melt extrusion. Eur J Pharm Biopharm. 2009;71(2):387–94.
Chokshi RJ, Sandhu HK, Iyer RM, Shah NH, Malick AW, Zia H. Characterization of physico-mechanical properties of indomethacin and polymers to assess their suitability for hot-melt extrusion processs as a means to manufacture solid dispersion/solution. J Pharm Sci. 2005;94(11):2463–74.
Verreck G, Six K, Van den Mooter G, Baert L, Peeters J, Brewster ME. Characterization of solid dispersions of itraconazole and hydroxypropylmethylcellulose prepared by melt extrusion—part I. Int J Pharm. 2003;251(1–2):165–74.
Six K, Berghmans H, Leuner C, Dressman J, Van Werde K, Mullens J, et al. Characterization of solid dispersions of itraconazole and hydroxypropylmethylcellulose prepared by melt extrusion, part II. Pharm Res. 2003;20(7):1047–54.
Tanno F, Nishiyama Y, Kokubo H, Obara S. Evaluation of hypromellose acetate succinate (HPMCAS) as a carrier in solid dispersions. Drug Dev Ind Pharm. 2004;30(1):9–17.
Friesen DT, Shanker R, Crew M, Smithey DT, Curatolo W, Nightingale J. Hydroxypropyl methylcellulose acetate succinate-based spray-dried dispersions: an overview. Mol Pharm. 2008;5(6):1003–19.
Ghosh I, Snyder J, Vippagunta R, Alvine M, Vakil R, Tong W-QT, et al. Comparison of HPMC based polymers performance as carriers for manufacture of solid dispersions using the melt extruder. Int J Pharm. 2011;419(1):12–9.
Chowdary K, Suresh BK. Dissolution, bioavailability and ulcerogenic studies on solid dispersions of indomethacin in water soluble cellulose polymers. Drug Dev Ind Pharm. 1994;20(5):799–813.
Onoue S, Sato H, Ogawa K, Kawabata Y, Mizumoto T, Yuminoki K, et al. Improved dissolution and pharmacokinetic behavior of cyclosporine A using high-energy amorphous solid dispersion approach. Int J Pharm. 2010;399(1):94–101.
Newa M, Bhandari KH, Li DX, Kwon T-H, Kim J, Yoo BK, et al. Preparation, characterization and in vivo evaluation of ibuprofen binary solid dispersions with poloxamer 188. Int J Pharm. 2007;343(1):228–37.
DiNunzio JC, Brough C, Miller DA, Williams III RO, McGinity JW. Applications of KinetiSol® Dispersing for the production of plasticizer free amorphous solid dispersions. Eur J Pharm Sci. 2010;40(3):179–87.
Djuris J, Nikolakakis I, Ibric S, Djuric Z, Kachrimanis K. Preparation of carbamazepine–Soluplus® solid dispersions by hot-melt extrusion, and prediction of drug–polymer miscibility by thermodynamic model fitting. Eur J Pharm Biopharm. 2013;84(1):228–37.
Rowe RC, Sheskey PJ, Owen SC, Association AP. Handbook of pharmaceutical excipients. London: Pharmaceutical press; 2006.
Alcohol M-P. Technical brochure. Sulzbach: Clariant GmgH; 1999.
Galindo-Rodriguez S, Allemann E, Fessi H, Doelker E. Physicochemical parameters associated with nanoparticle formation in the salting-out, emulsification-diffusion, and nanoprecipitation methods. Pharm Res. 2004;21(8):1428–39.
Abdel-Mottaleb MM, Mortada N, El-Shamy A, Awad G. Physically cross-linked polyvinyl alcohol for the topical delivery of fluconazole. Drug Dev Ind Pharm. 2009;35(3):311–20.
Bourges JL, Bloquel C, Thomas A, Froussart F, Bochot A, Azan F, et al. Intraocular implants for extended drug delivery: therapeutic applications. Adv Drug Deliv Rev. 2006;58(11):1182–202.
Davies NM, Fair SJ, Hadgraft J, Kellaway IW. Evaluation of mucoadhesive polymers in ocular drug delivery. I. Viscous solutions. Pharm Res. 1991;8(8):1039–43.
McDonald C, Kaye S, Figueiredo F, Macintosh G, Lockett C. A randomised, crossover, multicentre study to compare the performance of 0.1% (w/v) sodium hyaluronate with 1.4% (w/v) polyvinyl alcohol in the alleviation of symptoms associated with dry eye syndrome. Eye. 2002;16(5):601–7.
Winterton LC, Lally JM, Sentell KB, Chapoy LL. The elution of poly (vinyl alcohol) from a contact lens: the realization of a time release moisturizing agent/artificial tear. J Biomed Mater Res B Appl Biomater. 2007;80B(2):424–32.
Thanoo B, Sunny M, Jayakrishnan A. Controlled release of oral drugs from cross‐linked polyvinyl alcohol microspheres. J Pharm Pharmacol. 1993;45(1):16–20.
Sahoo SK, Panyam J, Prabha S, Labhasetwar V. Residual polyvinyl alcohol associated with poly (D, L-lactide-co-glycolide) nanoparticles affects their physical properties and cellular uptake. J Control Release. 2002;82(1):105–14.
Mu L, Feng S. A novel controlled release formulation for the anticancer drug paclitaxel (Taxol®): PLGA nanoparticles containing vitamin E TPGS. J Control Release. 2003;86(1):33–48.
Riis T, Bauer-Brandl A, Wagner T, Kranz H. pH-independent drug release of an extremely poorly soluble weakly acidic drug from multiparticulate extended release formulations. Eur J Pharm Biopharm. 2007;65(1):78–84.
Davaran S, Rashidi MR, Khandaghi R, Hashemi M. Development of a novel prolonged-release nicotine transdermal patch. Pharmacol Res. 2005;51(3):233–7.
DeMerlis C, Schoneker D. Review of the oral toxicity of polyvinyl alcohol (PVA). Food Chem Toxicol. 2003;41(3):319–26.
De Jaeghere W, De Beer T, Van Bocxlaer J, Remon J, Vervaet C.Hot-melt extrusion of polyvinyl alcohol for oral immediate release applications. Int J Pharm. 2015.
Keen JM, McGinity JW, Williams III RO. Enhancing bioavailability through thermal processing. Int J Pharm. 2013;450(1):185–96.
Miller DA, et al. KinetiSol: A New Processing Paradigm for Amorphous Solid Dispersion Systems. Drug Dev Deliv. 2012;12(9).
Gray V, Kelly G, Xia M, Butler C, Thomas S, Mayock S. The science of USP 1 and 2 dissolution: present challenges and future relevance. Pharm Res. 2009;26(6):1289–302.
Azarmi S, Roa W, Löbenberg R. Current perspectives in dissolution testing of conventional and novel dosage forms. Int J Pharm. 2007;328(1):12–21.
Galia E, Nicolaides E, Hörter D, Löbenberg R, Reppas C, Dressman J. Evaluation of various dissolution media for predicting in vivo performance of class I and II drugs. Pharm Res. 1998;15(5):698–705.
Hughey JR, DiNunzio JC, Bennett RC, Brough C, Miller DA, Ma H, et al. Dissolution enhancement of a drug exhibiting thermal and acidic decomposition characteristics by fusion processing: a comparative study of hot melt extrusion and kinetisol® dispersing. AAPS PharmSciTech. 2010;11(2):760–74.
Sarode AL, Sandhu H, Shah N, Malick W, Zia H. Hot melt extrusion (HME) for amorphous solid dispersions: predictive tools for processing and impact of drug–polymer interactions on supersaturation. Eur J Pharm Sci. 2013;48(3):371–84.
Trey SM, Wicks DA, Mididoddi PK, Repka MA. Delivery of itraconazole from extruded HPC films. Drug Dev Ind Pharm. 2007;33(7):727–35.
Nunes C, Mahendrasingam A, Suryanarayanan R. Quantification of crystallinity in substantially amorphous materials by synchrotron X-ray powder diffractometry. Pharm Res. 2005;22(11):1942–53.
Wang B, Mukataka S, Kokufuta E, Ogiso M, Kodama M. Viscometric, light scattering, and size-exclusion chromatography studies on the structural changes of aqueous poly(vinyl alcohol) induced by γ-ray irradiation. J Polym Sci B Polym Phys. 2000;38(1):214–21.
Hughey JR, Keen JM, Miller DA, Brough C, McGinity JW. Preparation and characterization of fusion processed solid dispersions containing a viscous thermally labile polymeric carrier. Int J Pharm. 2012;438(1):11–9.
Hutchinson JM, Kumar P. Enthalpy relaxation in polyvinyl acetate. Thermochim Acta. 2002;391(1):197–217.
Lu J, Wang T, Drzal LT. Preparation and properties of microfibrillated cellulose polyvinyl alcohol composite materials. Compos A: Appl Sci Manuf. 2008;39(5):738–46.
Chemburkar SR, Bauer J, Deming K, Spiwek H, Patel K, Morris J, et al. Dealing with the impact of ritonavir polymorphs on the late stages of bulk drug process development. Org Process Res Dev. 2000;4(5):413–7.
Stoclet G, Seguela R, Lefebvre J-M. Morphology, thermal behavior and mechanical properties of binary blends of compatible biosourced polymers: polylactide/polyamide11. Polymer. 2011;52(6):1417–25.
Richardson M, Flory P, Jackson J. Crystallization and melting of copolymers of polymethylene. Polymer. 1963;4:221–36.
Six K, Verreck G, Peeters J, Binnemans K, Berghmans H, Augustijns P, et al. Investigation of thermal properties of glassy itraconazole: identification of a monotropic mesophase. Thermochim Acta. 2001;376(2):175–81.
Rambali B, Verreck G, Baert L, Massart D. Itraconazole formulation studies of the melt-extrusion process with mixture design. Drug Devel Ind Pharm. 2003;29(6):641–52.
Acknowledgments
The authors wish to gratefully acknowledge the financial support of Merck Millipore.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: Feng Zhang and Michael A. Repka
Rights and permissions
About this article
Cite this article
Brough, C., Miller, D.A., Keen, J.M. et al. Use of Polyvinyl Alcohol as a Solubility-Enhancing Polymer for Poorly Water Soluble Drug Delivery (Part 1). AAPS PharmSciTech 17, 167–179 (2016). https://doi.org/10.1208/s12249-015-0458-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-015-0458-y