Abstract
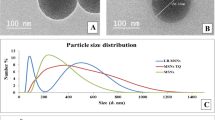
Breviscapine is used in the treatment of ischemic cerebrovascular diseases, but it has a low bioavailability in the brain due to its poor physicochemical properties and the activity of P-glycoprotein efflux pumps located at the blood–brain barrier. In the present study, breviscapine-loaded solid lipid nanoparticles (SLN) coated with polyethylene glycol (PEG) derivatives were formulated and evaluated for their ability to enhance brain bioavailability. The SLNs were either coated with polyethylene glycol (40) (PEG-40) stearate alone (Bre-GBSLN-PS) or a mixture of PEG-40 stearate and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-PEG2000 (DSPE-PEG2000) (Bre-GBSLN-PS-DSPE) and were characterized both in vitro and in vivo. The mean particle size, polydispersity index, and entrapment efficiency for Bre-GBSLN-PS and Bre-GBSLN-PS-DSPE were 21.60 ± 0.10 and 22.60 ± 0.70 nm, 0.27 ± 0.01 and 0.26 ± 0.04, and 46.89 ± 0.73% and 47.62 ± 1.86%, respectively. The brain pharmacokinetic parameters revealed that the brain bioavailability of breviscapine from the Bre-GBSLN-PS and Bre-GBSLN-PS-DSPE was significantly enhanced (p < 0.01) with the area under concentration–time curve (AUC) of 1.59 ± 0.39 and 1.42 ± 0.58 μg h/mL of breviscapine, respectively, in comparison to 0.11 ± 0.02 μg h/mL from the commercial breviscapine injection. The ratios of the brain AUC for scutellarin in comparison with the plasma scutellarin AUC for commercial breviscapine injection, Bre-GBSLN-PS, and Bre-GBSLN-PS-DSPE were 0.66%, 2.82%, and 4.51%, respectively. These results showed that though both SLN formulations increased brain uptake of breviscapine, Bre-GBSLN-PS-DSPE which was coated with a binary combination of PEG-40 stearate and DSPE-PEG2000 had a better brain bioavailability than Bre-GBSLN-PS. Thus, the coating of SLNs with the appropriate PEG derivative combination could improve brain bioavailability of breviscapine and can be a promising tool for brain drug delivery.








Similar content being viewed by others
References
Wang M, Xie C, Cai RL, Li XH, Luo XZ, Qi Y. Studies on antioxidant activities of breviscapine in the cell-free system. Am J Chinese Med. 2008;36(6):1199–207.
Cuzzocrea S, Riley DP, Caputi AP, Salvemini D. Antioxidant therapy: a new pharmacological approach in shock, inflammation, and ischemia/reperfusion injury. Pharmacol Rev. 2001;53(1):135–59.
Cao W, Liu W, Wu T, Zhong D, Liu G. Dengzhanhua preparations for acute cerebral infarction. Cochrane Database Syst Rev. 2008;4, CD005568. doi:10.1002/14651858.CD005568.pub2.
Qian L, Shen M, Tang H, Tang Y, Zhang L, Fu Y, et al. Synthesis and protective effect of scutellarein on focal cerebral ischemia/reperfusion in rats. Molecules. 2012;17(9):10667–74. doi:10.3390/molecules170910667.
Lv W, Guo J, Li J, Huang L, Ping Q. Distribution of liposomal breviscapine in brain following intravenous injection in rats. Int J Pharm. 2005;306(1–2):99–106. doi:10.1016/j.ijpharm.2005.09.012.
Sun H, Dai H, Shaik N, Elmquist WF. Drug efflux transporters in the CNS. Adv Drug Deliv Rev. 2003;55(1):83–105.
De Boer AG, Breimer DD. The blood–brain barrier: clinical implications for drug delivery to the brain. J R Coll Physicians Lond. 1994;28(6):502–6.
Schinkel AH. P-glycoprotein, a gatekeeper in the blood–brain barrier. Adv Drug Deliv Rev. 1999;36(2–3):179–94.
Liu M, Li H, Luo G, Liu Q, Wang Y. Pharmacokinetics and biodistribution of surface modification polymeric nanoparticles. Arch Pharm Res. 2008;31(4):547–54. doi:10.1007/s12272-001-1191-8.
She Z-Y, Ke X, Ping Q, Xu B-H, Chen B. Preparation of breviscapine nanosuspension and its pharmacokinetic behavior in rats. Chin J Nat Med. 2007;5(1):50–5.
Dingler A, Runge S, Muller RH. SLN (solid lipid nanoparticles) as drug carrier for an IV administration of poorly water soluble drugs. Eur J Pharm Sci. 1996;4(1001):132. doi:10.1016/s0928-0987(97)86382-8.
Silva GA. Nanotechnology approaches to crossing the blood–brain barrier and drug delivery to the CNS. BMC Neurosci. 2008;9 Suppl 3:S4. doi:10.1186/1471-2202-9-S3-S4.
Madan J, Pandey RS, Jain V, Katare OP, Chandra R, Katyal A. Poly (ethylene)-glycol conjugated solid lipid nanoparticles of noscapine improve biological half-life, brain delivery and efficacy in glioblastoma cells. Nanomedicine. 2013;9(4):492–503. doi:10.1016/j.nano.2012.10.003.
Wang SW, Monagle J, McNulty C, Putnam D, Chen H. Determination of P-glycoprotein inhibition by excipients and their combinations using an integrated high-throughput process. J Pharm Sci. 2004;93(11):2755–67. doi:10.1002/jps.20183.
Zhu S, Huang R, Hong M, Jiang Y, Hu Z, Liu C, et al. Effects of polyoxyethylene (40) stearate on the activity of P-glycoprotein and cytochrome P450. Eur J Pharm Sci. 2009;37(5):573–80. doi:10.1016/j.ejps.2009.05.001.
Sawchuk RJ, Elmquist WF. Microdialysis in the study of drug transporters in the CNS. Adv Drug Deliv Rev. 2000;45(2–3):295–307.
Subedi RK, Kang KW, Choi HK. Preparation and characterization of solid lipid nanoparticles loaded with doxorubicin. Eur J Pharm Sci. 2009;37(3–4):508–13. doi:10.1016/j.ejps.2009.04.008.
Oyewumi MO, Mumper RJ. Gadolinium-loaded nanoparticles engineered from microemulsion templates. Drug Dev Ind Pharm. 2002;28(3):317–28. doi:10.1081/ddc-120002847.
Yue PF, Lu XY, Zhang ZZ, Yuan HL, Zhu WF, Zheng Q, et al. The study on the entrapment efficiency and in vitro release of puerarin submicron emulsion. AAPS PharmSciTech. 2009;10(2):376–83. doi:10.1208/s12249-009-9216-3.
Shimada K, Miyagishima A, Sadzuka Y, Nozawa Y, Mochizuki Y, Ohshima H, et al. Determination of the thickness of the fixed aqueous layer around polyethyleneglycol-coated liposomes. J Drug Target. 1995;3(4):283–9. doi:10.3109/10611869509015957.
Peracchia MT, Vauthier C, Passirani C, Couvreur P, Labarre D. Complement consumption by poly(ethylene glycol) in different conformations chemically coupled to poly(isobutyl 2-cyanoacrylate) nanoparticles. Life Sci. 1997;61(7):749–61.
Litman T, Zeuthen T, Skovsgaard T, Stein WD. Structure-activity relationships of P-glycoprotein interacting drugs: kinetic characterization of their effects on ATPase activity. Biochim Biophys Acta. 1997;1361(2):159–68.
Lonnroth P, Jansson PA, Smith U. A microdialysis method allowing characterization of intercellular water space in humans. Am J Physiol. 1987;253(2 Pt 1):E228–31.
Wang Y, Wong SL, Sawchuk RJ. Microdialysis calibration using retrodialysis and zero-net flux: application to a study of the distribution of zidovudine to rabbit cerebrospinal fluid and thalamus. Pharm Res. 1993;10(10):1411–9.
Zhong D, Yang B, Chen X, Li K, Xu J. Determination of scutellarin in rat plasma by high-performance liquid chromatography with ultraviolet detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;796(2):439–44.
Burra M, Jukanti R, Janga KY, Sunkavalli S, Velpula A, Ampati S, et al. Enhanced intestinal absorption and bioavailability of raloxifene hydrochloride via lyophilized solid lipid nanoparticles. Adv Powder Technol. 2013;24(1):393–402. doi:10.1016/j.apt.2012.09.002.
Li S, Ji Z, Zou M, Nie X, Shi Y, Cheng G. Preparation, characterization, pharmacokinetics and tissue distribution of solid lipid nanoparticles loaded with tetrandrine. AAPS PharmSciTech. 2011;12(3):1011–8. doi:10.1208/s12249-011-9665-3.
Juillerat-Jeanneret L. The targeted delivery of cancer drugs across the blood–brain barrier: chemical modifications of drugs or drug-nanoparticles? Drug Discov Today. 2008;13(23–24):1099–106.
Muller RH, Mader K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery—a review of the state of the art. Eur J Pharm Biopharm. 2000;50(1):161–77.
Peltonen L, Aitta J, Hyvonen S, Karjalainen M, Hirvonen J. Improved entrapment efficiency of hydrophilic drug substance during nanoprecipitation of poly(l)lactide nanoparticles. AAPS PharmSciTech. 2004;5(1):E16. doi:10.1208/pt050116.
Cao F, Guo JX, Ping QN, Liao ZG. Prodrugs of scutellarin: ethyl, benzyl and N,N-diethylglycolamide ester synthesis, physicochemical properties, intestinal metabolism and oral bioavailability in the rats. Eur J Pharm Sci. 2006;29(5):385–93. doi:10.1016/j.ejps.2006.07.007.
Tsai MJ, Huang YB, Wu PC, Fu YS, Kao YR, Fang JY, et al. Oral apomorphine delivery from solid lipid nanoparticles with different monostearate emulsifiers: pharmacokinetic and behavioral evaluations. J Pharm Sci. 2011;100(2):547–57. doi:10.1002/jps.22285.
Souto EB, Muller RH. Investigation of the factors influencing the incorporation of clotrimazole in SLN and NLC prepared by hot high-pressure homogenization. J Microencapsul. 2006;23(4):377–88. doi:10.1080/02652040500435295.
Dong X, Mattingly CA, Tseng MT, Cho MJ, Liu Y, Adams VR, et al. Doxorubicin and paclitaxel-loaded lipid-based nanoparticles overcome multidrug resistance by inhibiting P-glycoprotein and depleting ATP. Cancer Res. 2009;69(9):3918–26. doi:10.1158/0008-5472.can-08-2747.
Kopecka J, Salzano G, Campia I, Lusa S, Ghigo D, De Rosa G, et al. Insights in the chemical components of liposomes responsible for P-glycoprotein inhibition. Nanomedicine. 2013. doi:10.1016/j.nano.2013.06.013.
Cauda V, Argyo C, Bein T. Impact of different PEGylation patterns on the long-term bio-stability of colloidal mesoporous silica nanoparticles. J Mater Chem. 2010;20(39):8693–9.
Sugiyama I, Sadzuka Y. Characterization of novel mixed polyethyleneglycol modified liposomes. Biol Pharm Bull. 2007;30(1):208–11.
Shono Y, Nishihara H, Matsuda Y, Furukawa S, Okada N, Fujita T, et al. Modulation of intestinal P-glycoprotein function by cremophor EL and other surfactants by an in vitro diffusion chamber method using the isolated rat intestinal membranes. J Pharm Sci. 2004;93(4):877–85. doi:10.1002/jps.20017.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (81001645), Program for New Century Excellent Talents in University (NCET-12-1068), Tianjin Natural Science Foundation (12JCYBJC18700), and the National Key Technology Research and Development Program of China (2012ZX09304007).
Author information
Authors and Affiliations
Corresponding author
Additional information
Zhidong Liu and Chukwunweike Ikechukwu Okeke contributed equally to this work.
Rights and permissions
About this article
Cite this article
Liu, Z., Okeke, C.I., Zhang, L. et al. Mixed Polyethylene Glycol-Modified Breviscapine-Loaded Solid Lipid Nanoparticles for Improved Brain Bioavailability: Preparation, Characterization, and In Vivo Cerebral Microdialysis Evaluation in Adult Sprague Dawley Rats. AAPS PharmSciTech 15, 483–496 (2014). https://doi.org/10.1208/s12249-014-0080-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-014-0080-4