Abstract
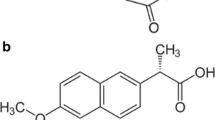
Sink conditions used in dissolution tests lead to rapid dissolution rates for nanosuspensions, causing difficulties in discriminating dissolution profiles between different formulations. Here, non-sink conditions were studied for the dissolution testing of poorly water-soluble drug nanosuspensions. A mathematical model for polydispersed particles was established to clarify dissolution mechanisms. The dissolution of nanosuspensions with either a monomodal or bimodal size distribution was simulated. In the experimental part, three different particle sizes of indomethacin nanosuspensions were prepared by the wet milling technique. The effects of the dissolution medium pH and agitation speed on dissolution rate were investigated. The dissolution profiles in sink and non-sink conditions were obtained by changing the ratio of sample amount to the saturation solubility. The results of the simulations and experiments indicated that when the sample amount was increased to the saturation solubility of drug, the slowest dissolution rate and the best discriminating dissolution profiles were obtained. Using sink conditions or too high amount of the sample will increase the dissolution rate and weaken the discrimination between dissolution profiles. Furthermore, the low solubility by choosing a proper pH of the dissolution medium was helpful in getting discriminating dissolution profiles, whereas the agitation speed appeared to have little influence on the dissolution profiles. This discriminatory method is simple to perform and can be potentially used in any nanoproduct development and quality control studies.








Similar content being viewed by others
REFERENCES
Sharma P, Garg S. Pure drug and polymer based nanotechnologies for the improved solubility, stability, bioavailability and targeting of anti-HIV drugs. Adv Drug Deliv Rev. 2010;62:491–502.
Chen H, Khemtong C, Yang X, Chang X, Gao J. Nanonization strategies for poorly water-soluble drugs. Drug Discov Today. 2011;16:354–60.
Date AA, Patravale VB. Current strategies for engineering drug nanoparticles. Curr Opin Colloid Interface Sci. 2004;9:222–35.
Müller RH, Keck CM. Twenty years of drug nanocrystals: where are we, and where do we go? Eur J Pharm Biopharm. 2012;80:1–3.
Van Eerdenbrugh B, Van den Mooter G, Augustijns P. Top-down production of drug nanocrystals: nanosuspension stabilization, miniaturization and transformation into solid products. Int J Pharm. 2008;364(1):64–75.
Müller RH, Peters K. Nanosuspensions for the formulation of poorly soluble drugs I. Preparation by a size-reduction technique. Int J Pharm. 1998;160(2):229–37.
Krishna R, Yu L. Biopharmaceutics applications in drug development. 3rd ed. New York: Springer; 2008.
Liu P, Rong X, Laru J, Van Veen B, Kiesvaara J, Hirvonen J, et al. Nanosuspensions of poorly soluble drugs: preparation and development by wet milling. Int J Pharm. 2011;411:215–22.
Ambrus R, Kocbek P, Kristl J, Sibanc R, Rajkó R, Szabó-Révész P. Investigation of preparation parameters to improve the dissolution of poorly water-soluble meloxicam. Int J Pharm. 2009;381(2):153–9.
Ganta S, Paxton JW, Baguley BC, Garg S. Formulation and pharmacokinetic evaluation of an asulacrine nanocrystalline suspension for intravenous delivery. Int J Pharm. 2009;367:179–86.
Sylvestre JP, Tang MC, Furtos A, Leclair G, Meunier M, Leroux JC. Nanonization of megestrol acetate by laser fragmentation in aqueous milieu. J Contr Release. 2011;149(3):273–80.
Tucker CJ. Real time monitoring of small particle dissolution by way of light scattering. US Patent; 2004.
Crisp MT, Tucker CJ, Rogers TL, Williams III RO, Johnston KP. Turbidimetric measurement and prediction of dissolution rates of poorly soluble drug nanocrystals. J Contr Release. 2007;117(3):351–9.
Peeters K, De Maesschalck R, Bohets H, Vanhoutte K, Nagels L. In situ dissolution testing using potentiometric sensors. Eur J Pharm Sci. 2008;34:243–9.
Kayaert P, Li B, Jimidar I, Rombaut P, Ahssini F, Van den Mooter G. Solution calorimetry as an alternative approach for dissolution testing of nanosuspensions. Eur J Pharm Biopharm. 2010;76(3):507–13.
Bhardwaj U, Burgess DJ. A novel USP apparatus 4 based release testing method for dispersed systems. Int J Pharm. 2010;388:287–94.
Siewert M, Dressman J, Brown CK, Shah VP. FIP/AAPS guidelines to dissolution/in vitro release testing of novel/special dosage forms. AAPS PharmSciTech. 2003;4(1):article 7.
Fogler HS. Elements of chemical reaction engineering. 3rd ed. Upper Saddle River: Prentice Hall PTR; 1999.
Frössling N. Über die Verdunstung fallender Tropfen. Gerl Beitr Geophys. 1938;52:170–216.
Higuchi WI, Hiestand EN. Dissolution rates of finely divided drug powders I. Effect of a distribution of particle sizes in a diffusion-controlled process. J Pharm Sci. 1963;52:67–71.
Hintz RJ, Johnson KC. The effect of particle size distribution on dissolution rate and oral absorption. Int J Pharm. 1989;51:9–17.
Wang Y, Abrahamsson B, Lindfors L, Brasseur JG. Comparison and analysis of theoretical models for diffusion-controlled dissolution. Mol Pharm. 2012;9(5):1052–66.
Laaksonen T, Liu P, Rahikkala A, Peltonen L, Kauppinen EI, Hirvonen J, et al. Intact nanoparticulate indomethacin in fast-dissolving carrier particles by combined wet milling and aerosol flow reactor methods. Pharm Res. 2011;28(10):2403–11.
Cerdeiraa AM, Mazzottib M, Ganderc B. Miconazole nanosuspensions: influence of formulation variables on particle size reduction and physical stability. Int J Pharm. 2010;396:210–8.
Peltonen L, Hirvonen J. Pharmaceutical nanocrystals by nanomilling: critical process parameters, particle fracturing and stabilization methods. J Pharm Pharmacol. 2010;62(11):1569–79.
Dolenc A, Kristl J, Baumgartner S, Planinsek O. Advantages of celecoxib nanosuspension formulation and transformation into tablets. Int J Pharm. 2009;376:204–12.
Jamzad S, Fassihi R. Role of surfactant and pH on dissolution properties of fenofibrate and glipizide—a technical note. AAPS PharmSciTech. 2006;7(2):Article 33.
Nokhodchi A, Javadzadeh Y, Siahi-Shadbad MR, Barzegar-Jalali M. The effect of type and concentration of vehicles on the dissolution rate of a poorly soluble drug (indomethacin) from liquisolid compacts. J Pharm Pharm Sci. 2005;8(1):18–25.
Agata Y, Iwao Y, Miyagishima A, Itai S. Novel mathematical model for predicting the dissolution profile of spherical particles under non-sink conditions. Chem Pharm Bull. 2010;58(4):511–5.
Anhalt K, Geissler S, Harms M, Weigandt M, Fricker G. Development of a new method to assess nanocrystal dissolution based on light scattering. Pharm Res. 2012;29(10):2887–901.
Dolenc A, Kristl J, Baumgartner S, Planinšek O. Advantages of celecoxib nanosuspension formulation and transformation into tablets. Int J Pharm. 2009;376:204–12.
Van Eerdenbrugh B, Froyen L, Van Humbeeck J, Martens JA, Augustijnsa P, Van den Mooter G. Drying of crystalline drug nanosuspensions—the importance of surface hydrophobicity on dissolution behavior upon redispersion. Eur J Pharm Sci. 2008;35:127–35.
Limnell T, Heikkilä T, Santos HA, Sistonen S, Hellstén S, Laaksonen T, et al. Physicochemical stability of high indomethacin payload ordered mesoporous silica MCM-41 and SBA-15 microparticles. Int J Pharm. 2011;416(1):242–51.
Heng D, Cutler DJ, Chan HK, Yun J, Raper JA. What is a suitable dissolution method for drug nanoparticles? Pharm Res. 2008;25(7):1696–701.
Gupta A, Gaud RS, Ganga S. Development of discriminating dissolution method for an insoluble drug: nisoldipine. Int J Pharm Tech Res. 2010;2(1):931–9.
Sugano K. Theoretical comparison of hydrodynamic diffusion layer models used for dissolution simulation in drug discovery and development. Int J Pharm. 2008;363:73–7.
ACKNOWLEDGMENTS
We acknowledge the financial support from Orion Pharma and China Scholarship Council. We thank Roy Siddall for providing language help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, P., De Wulf, O., Laru, J. et al. Dissolution Studies of Poorly Soluble Drug Nanosuspensions in Non-sink Conditions. AAPS PharmSciTech 14, 748–756 (2013). https://doi.org/10.1208/s12249-013-9960-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-013-9960-2