Abstract
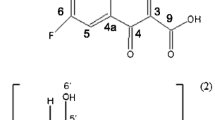
β-cyclodextrin (βCD) and methyl-β-cyclodextrin (MβCD) complexes with sulfamethazine (SMT) were prepared and characterized by different experimental techniques, and the effects of βCD and MβCD on drug solubility were assessed via phase-solubility analysis. The phase-solubility diagram for the drug showed an increase in water solubility, with the following affinity constants calculated: 40.4 ± 0.4 (pH 2.0) and 29.4 ± 0.4 (pH 8.0) M−1 with βCD and 56 ± 1 (water), 39 ± 3 (pH 2.0) and 39 ± 5 (pH 8.0) M−1 with MβCD. According to 1H NMR and 2D NMR spectroscopy, the complexation mode involved the aromatic ring of SMT included in the MβCD cavity. The complexes obtained in solid state by freeze drying were characterized by Fourier transform infrared spectroscopy, scanning electron microscopy, and thermal analysis. The amorphous complexes obtained in this study may be useful in the preparation of pharmaceutical dosage forms of SMT.








Similar content being viewed by others
REFERENCES
Mayersohn M. Principles of drug absorption. In: Banker GS, Rhodes CT, editors. Modern pharmaceutics. New York: Marcel Dekker; 2002. p. 23–66.
Budavari S, O’Neil MJ, Smith A, Heckelman PE. The Merck Index. 13th ed. Whitehouse Station: Merck and Co; 2001.
Papastephanou C, Frantz M. Sulfamethazine. In: Florey K, editor. Analytical profiles of drug substances. New York: Academic; 1982. p. 401–22.
Reguera C, Ortiz MC, Herrero A, Sarabia LA. Optimization of a FIA system with amperometric detection by means of a desirability function: Determination of sulfadiazine, sulfamethazine and sulfamerazine in milk. Talanta. 2008;75:274–83.
Zhang H, Zhang Y, Wang S. Development of flow-through and dip-stick immunoassays for screening of sulfonamide residues. J ImmunOL Methods. 2008;337:1–6.
Tommasino J-B, Renaud FNR, Luneau D, Pilet G. Multi-biofunctional complexes combining antiseptic copper(II) with antibiotic sulfonamide ligands: Structural, redox and antibacterial study. Polyhedron. 2011;30:1663–70.
Hossain GMG, Amoroso AJ, Banu A, Malik KMA. Syntheses and characterisation of mercury complexes of sulfadiazine, sulfamerazine and sulfamethazine. Polyhedron. 2007;26:967–74.
Gamba V, Terzano C, Fioroni L, Moretti S, Dusi G, Galarini R. Development and validation of a confirmatory method for the determination of sulphonamides in milk by liquid chromatography with diode array detection. Anal Chim Acta. 2009;637:18–23.
Ramos Payán M, López MÁB, Fernández-Torres R, Navarro MV, Mochón MC. Hollow fiber-based liquid phase microextraction (HF-LPME) for a highly sensitive HPLC determination of sulfonamides and their main metabolites. J Chromatogr B. 2011;879:197–204.
Brewster ME, Loftsson T. Cyclodextrins as pharmaceutical solubilizers. Adv Drug Deliv Rev. 2007;59:645–66.
Loftsson T, Brewster ME. Pharmaceutical applications of cyclodextrins: basic science and product development. J Pharm Pharmacol. 2010;62:1607–21.
Loftsson T, Brewster ME. Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J Pharm Sci. 1996;85:1017–25.
Fromming KH, Szejtli J. Cyclodextrins in pharmacy. Dordrecht: Kluwer Academic; 1994.
Delrivo A, Zoppi A, Longhi MR. Interaction of sulfadiazine with cyclodextrins in aqueous solution and solid state. Carbohydr Polym. 2012;87:1980–8.
Zoppi A, Quevedo MA, Delrivo A, Longhi MR. Complexation of sulfonamides with beta-cyclodextrin studied by experimental and theoretical methods. J Pharm Sci. 2010;99:3166–76.
Granero GE, Garnero C, Longhi MR. The effect of pH and triethanolamine on sulfisoxazole complexation with hydroxypropyl-β-cyclodextrin. Eur J Pharm Sci. 2003;20:285–93.
Garnero C, Aiassa V, Longhi M. Sulfamethoxazole:hydroxypropyl-β-cyclodextrin complex: preparation and characterization. J Pharm Biomed Anal. 2012;63:74–9.
Granero GE, Maitre MM, Garnero C, Longhi MR. Synthesis, characterization and in vitro release studies of a new acetazolamide-HP-β-CD-TEA inclusion complex. Eur J Med Chem. 2008;43:464–70.
Palma SD, Tartara LI, Quinteros D, Allemandi DA, Longhi MR, Granero GE. An efficient ternary complex of acetazolamide with HP-ß-CD and TEA for topical ocular administration. J Control Release. 2009;138:24–31.
Sourbaji M, Pintye-Hódi K, Novák CS, Szabó-Révész P, Kása Jr P, Erõs I. A study of sulfadimidine-β-cyclodextrin mixtures. J Incl Phenom. 2000;37:299–307.
Higuchi T, Connors KA. Phase-solubility techniques. Adv Anal Chem Instrum. 1965;4:117–212.
Ge X, Huang Z, Tian S, Huang Y, Zeng C. Complexation of carbendazim with hydroxypropyl-β-cyclodextrin to improve solubility and fungicidal activity. Carbohydr Polym. 2012;89:208–12.
Salustio PJ, Feio G, Figueirinhas JL, Pinto JF, Cabral Marques HM. The influence of the preparation methods on the inclusion of model drugs in a beta-cyclodextrin cavity. Eur J Pharm Biopharm. 2009;71:377–86.
Naidu NB, Chowdary KP, Murthy KV, Satyanarayana V, Hayman AR, Becket G. Physicochemical characterization and dissolution properties of meloxicam-cyclodextrin binary systems. J Pharm Biomed Anal. 2004;35:75–86.
ACKNOWLEDGMENTS
The authors thank the Fondo para la Investigación Científica y Tecnológica (FONCYT) Préstamo BID PICT 1376, the Secretaría de Ciencia y Técnica de la Universidad Nacional de Córdoba (SECyT), and the Consejo Nacional de Investigaciones Científicas y Tecnológicas de la Nación (CONICET) for financial support. We also thank Ferromet S.A. (agent of Roquette in Argentina) for its donation of βCD and MβCD. We are grateful to Dr. Gloria Bonetto for NMR measurements and for her helpful discussion of the 1H NMR spectra, and to Dr. Paul Hobson, native English speaker, for revision of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zoppi, A., Delrivo, A., Aiassa, V. et al. Binding of Sulfamethazine to β-cyclodextrin and Methyl-β-cyclodextrin. AAPS PharmSciTech 14, 727–735 (2013). https://doi.org/10.1208/s12249-013-9958-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-013-9958-9