Abstract
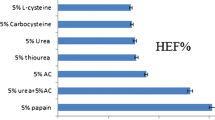
Onychomycosis is associated with the cutaneous fungal infection of the nail and the nail folds (skin surrounding the nail). It is therefore important to target drug delivery into the nail folds along with nail plate and the nail bed. Systematic and strategic selection of the penetration enhancers specific for the skin and the nail is discussed. Twelve penetration enhancers were screened for their ability to improve solubility, in vitro nail penetration, in vitro skin permeation, and in vitro skin penetration of the antifungal drug ciclopirox olamine. In contrast to transdermal drug delivery, the main selection criteria for skin penetration enhancer in topical drug delivery were increased drug accumulation in the epidermis and minimal permeation across the skin. Thiourea improved the solubility and nail penetration of ciclopirox olamine. It also showed enhancement in the transungual diffusion of the drug. Propylene glycol showed a 12-fold increase in solubility and 3-fold increase in epidermal accumulation of ciclopirox olamine, while minimizing the transdermal movement of the drug. Thiourea was the selected nail permeation enhancer and propylene glycol was the selected skin penetration enhancer of ciclopirox olamine. A combination of the selected enhancers was also explored for its effect on drug delivery to the nail and nail folds. The enhancer combination reduced the penetration of ciclopirox in the skin and also the permeation through the nail. The proposed preformulation strategy helps to select appropriate enhancers for optimum topical delivery and paves way towards an efficient topical formulation for passive transungual drug delivery.






Similar content being viewed by others
REFERENCES
Baran R, Hay RJ, Tosti A, Haneke E. A new classification of onychomycosis. Br J Dermatol. 1998;139:567–71.
Drake LA. Impact of onychomycosis on quality of life. J Am Podiatr Med Assoc. 1997;87(11):507–11.
Drake LA, Scher RK, Smith EB, Faich GA, Smith SL, Hong JJ, et al. Effect of onychomycosis on quality of life. J Am Acad Dermatol. 1998;38:702–4.
Lubeck DP. Measuring health-related quality of life in onychomycosis. J Am Acad Dermatol. 1998;38:S64–8.
Drake LA, Patrick DL, Fleckman P, André J, Baran R, Haneke E, et al. The impact of onychomycosis on quality of life: development of an international onychomycosis-specific questionnaire to measure patient quality of life. J Am Acad Dermatol. 1999;41(2 Part1):189–96.
Turner RR, Testa MA. Measuring the impact of onychomycosis on patient quality of life. Qual Life Res. 2000;9(1):39–53.
Gregory N. Special patient populations: onychomycosis in the HIV-positive patient. J Am Acad Dermatol. 1996;35(3 part 2):S13–6.
Moreno-Coutiño G, Arenas R, Reyes-Terán G. Clinical presentation of onychomycosis in HIV/AIDS: a review of 280 Mexican cases. Indian J Dermatol. 2011;56(1):120–1.
Gupta AK, Taborda P, Taborda V, Gilmour J, Rachlis A, Salit I, et al. Epidemiology and prevalence of onychomycosis in HIV-positive individuals. Int J Dermatol. 2000;39:749–53.
Levy LA. Epidemiology of onychomycosis in special-risk populations. J Am Podiatr Med Assoc. 1997;87(12):546–50.
Gupta AK, Lynde CW, Jain, Sibbald RG, Elewski BE, Daniel CR, et al. A higher prevalence of onychomycosis in psoriatics compared with non-psoriatics: a multicentre study. Br J Dermatol. 1997;136:786–9.
Szepietowski JC, Salomon J. Do fungi play a role in psoriatic nails? Mycoses. 2007;50(6):437–42.
Winston JA, Miller JL. Treatment of onychomycosis in diabetic patients. Clin Diabetes. 2006;24(4):160–6.
Gupta AK, Fleckman P, Baran R. Ciclopirox nail lacquer topical solution 8% in the treatment of toenail onychomycosis. J Am Acad Dermatol. 2000;43:S70–80.
Monti D, Saccomani L, Chetoni P, Burgalassi S, Saettone MF, Mailland F. In vitro transungual permeation of ciclopirox from a hydroxypropyl chitosan-based, water-soluble nail lacquer. Drug Dev Ind Pharm. 2005;31:11–7.
Johnson L. Dermatophytes—the skin eater. Mycologist. 2003;17(4):147–9.
Subissi A, Monti D, Togni G, Maillan F. Ciclopirox: recent nonclinical and clinical data relevant to its use as a topical antimycotic agent. Drugs. 2010;70(16):2133–52.
Khengar R, Jones S, Turner R, Forbes B, Brown M. Nail swelling as a pre-formulation screen for the selection and optimisation of ungual penetration enhancers. Pharm Res. 2007;24(12):2207–12.
Williams A, Barry B. Penetration enhancers. Adv Drug Deliv Rev. 2004;56:603–18.
Escarrone AL, Bittencourt C, Laporta L, dos Santos M, Primel E, Caldas S. LC–UV method with pre-column derivatization for the determination of ciclopirox olamine in raw material and topical solution. Chromatographia. 2008;67:967–71.
Lehr K-H, Damm P. Quantification of ciclopirox by high-performance liquid chromatography after pre-column derivatization. J Chromatogr. 1985;339:451–6.
Myoung Y, Choi H-K. Permeation of ciclopirox across porcine hoof membrane: effect of pressure sensitive adhesives and vehicles. Eur J Pharm Sci. 2003;20:319–25.
Frank JD, Manson JM, Cartwright ME. Separation of epidermis from dermis in the rhesus monkey. Exp Dermatol. 1995;4(2):89–92.
Kassis V, SØndergaard J. Heat separation of normal human skin for epidermal and dermal prostaglandin analysis. Arch Dermatol Res. 1982;273:301–6.
Murthy SN, Vaka SR, Sammeta SM, Nair AB. TranScreen-N: method for rapid screening of trans-ungual drug delivery enhancers. J Pharm Sci. 2009;98(11):4264–71.
Manda P, Sammeta SM, Repka MA, Murthy N. Iontophoresis across the proximal nail fold to target drugs to the nail matrix. J Pharm Sci. 2012;101(7):2392–7.
Kokjohn K, Bradley M, Griffiths B, Ghannoum M. Evaluation of in vitro activity of ciclopirox olamine, butenafine HCl and econazole nitrate against dermatophytes, yeasts and bacteria. Int J Dermatol. 2003;42(S1):11–7.
Chouhan P, Saini TR. Hydration of nail plate: a novel screening model for transungual drug permeation enhancers. Int J Pharm. 2012;436(1–2):179–82.
Traynor MJ, Turner RB, Evans CR, Khengar RH, Jones SA, Brown MB. Effect of a novel penetration enhancer on the ungual permeation of two antifungal agents. J Pharm Pharmacol. 2010;62(6):730–7.
Gupta AK, Kohli Y. In vitro susceptibility testing of ciclopirox, terbinafine, ketoconazole and itraconazole against dermatophytes and nondermatophytes, and in vitro evaluation of combination antifungal activity. Br J Dermatol. 2003;149(2):296–305.
Shehata AS, Mukherjee PK, Ghannoum MA. Comparison between the standardized clinical and laboratory standards institute M38-A2 method and a 2,3-Bis(2-methoxy-4-nitro-5-[(sulphenylamino)carbonyl])-2H-tetrazolium hydroxide based method for testing antifungal susceptibility of dermatophytes. J Clin Microbiol. 2008;46(11):3668–71.
Singh J, Zaman M, Gupta AK. Evaluation of microdilution and disk diffusion methods for antifungal susceptibility testing of dermatophytes. Med Mycol. 2007;45(7):595–602.
Schaller M, Borelli C, Berger U, Walker B, Schmidt S, Weindl G, et al. Susceptibility testing of amorolfine, bifonazole and ciclopirox olamine against Trichophyton rubrum in an in vitro model of dermatophyte nail infection. Med Mycol. 2009;47:753–8.
Kumar S, Malick AW, Meleer NM, Mouskountakis JD, Behl CR. Studies of in vitro skin permeation and retention of a leukotriene antagonist from topical vehicles with a hairless guinea pig model. J Pharm Sci. 1992;81(7):631–4.
Thomas NS, Panchagnula R. Transdermal delivery of zidovudine: effect of vehicles on permeation across rat skin and their mechanism of action. Eur J Pharm Sci. 2003;18(1):71–9.
Touitou E, Abed L. Effect of propylene glycol, Azone and n-decylmethyl sulphoxide on skin permeation kinetics of 5-fluorouracil. Int J Pharm. 1985;27:89–98.
Bonina FP, Montenegro L. Penetration enhancer effects on in vitro of heparin sodium percutaneous absorption salt. Int J Pharm. 1992;82:171–7.
Lorenzetti OJ. Propylene glycol gel vehicles. Cutis. 1979;23:747–50.
Ritschel W, Panchagnula R, Stemmer K, Ashraf M. Development of an intracutaneous depot of drugs. Skin Pharmacol. 1991;4:235–45.
Puglia C, Bonina F, Trapani G, Franco M, Ricci M. Evaluation of in vitro percutaneous absorption of lorazepam and clonazepam from hydro-alcoholic gel formulations. Int J Pharm. 2001;228(1–2):79–87.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Palliyil, B., Lebo, D.B. & Patel, P.R. A Preformulation Strategy for the Selection of Penetration Enhancers for a Transungual Formulation. AAPS PharmSciTech 14, 682–691 (2013). https://doi.org/10.1208/s12249-013-9954-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-013-9954-0