Abstract
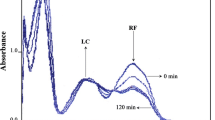
The photolysis of riboflavin (RF) in the presence of acetate buffer (pH 3.8–5.6) and carbonate buffer (pH 9.2–10.8) has been studied using a multicomponent spectrophotometric method for the simultaneous assay of RF and its photoproducts. Acetate and carbonate buffers have been found to catalyze the photolysis reaction of RF. The apparent first-order rate constants for the acetate-catalyzed reaction range from 0.20 to 2.86 × 10−4 s−1 and for the carbonate-catalyzed reaction from 3.33 to 15.89 × 10−4 s−1. The second-order rate constants for the interaction of RF with the acetate and the carbonate ions range from 2.04 to 4.33 × 10−4 M−1 s−1 and from 3.71 to 11.80 × 10−4 M−1 s−1, respectively. The k-pH profile for the acetate-catalyzed reaction is bell shaped and for the carbonate-catalyzed reaction a steep curve. Both HCO −3 and CO 2 −3 ions are involved in the catalysis of the photolysis reaction in alkaline solution. The rate constants for the HCO −3 and CO 2 −3 ions catalyzed reactions are 0.72 and 1.38 × 10−3 M−1 s−1, respectively, indicating a major role of CO 2 −3 ions in the catalysis reaction. The loss of RF fluorescence in acetate buffer suggests an interaction between RF and acetate ions to promote the photolysis reaction. The optimum stability of RF solutions is observed in the pH range 5–6, which is suitable for pharmaceutical preparations.



Similar content being viewed by others
References
Lachman L, DeLuca P, Askers MJ. Kinetic principles and stability testing. In: Lachman L, Lieberman HA, Kanig JL, editors. The theory and practice of industrial pharmacy. 2nd ed. Philadelphia: Lea & Febiger; 1986. p. 760–803.
Connors KA, Amidon GL, Stella VJ. Chemical stability of pharmaceuticals a handbook for the pharmacist. 2nd ed. New York: Wiley; 1986.
Laidler KJ. Chemical kinetics. 3rd ed. New York: Harper & Row; 1987. p. 384–99.
Carstensen JT. Catalysis, complexation and photolysis. In: Carstensen JT, Rhodes CT, editors. Drug stability principles and practices. 3rd ed. New York: Marcel Dekker; 2000. p. 73–84.
Yoshioka S, Stella VJ. Stability of drugs and dosage forms. New York: Klumer Academic/Plenuim Publisher; 2000. p. 97–9.
Florence AT, Attwood D. Drug stability. In: Physiochemical principles of pharmacy. 4th ed. London: Pharmaceutical Press; 2006. p. 93–138.
Sinko PJ. Chemical kinetics and stability. In: Martin’s physical pharmacy and pharmaceutical sciences. 5th ed. Philadelphia, PA: Lippincott Willams & Wilkins, 2006, p. 416–20.
British Pharmacopoeia, London: Her Majesty’s Stationary Office; 2012, Electronic version.
United States Pharmacopoeia, 30-National Formulary 25. Rockville, MD: United States Pharmacopoeial Convention, Inc., 2013. Electronic version.
Ahmad I, Fasiullah Q, Noor A, Ansari IA, Ali QNM. Photolysis of riboflavin in aqueous solution: a kinetic study. Int J Pharm. 2004;280:199–208.
Halwer M. The photochemistry of riboflavin and related compounds. J Am Chem Soc. 1951;73:4870–4.
Holmstrom B, Oster G. Riboflavin as an electron donor in photochemical reactions. J Am Chem Soc. 1961;83:1867–71.
Schuman Jorns M, Sehollnhammer G, Hemmerich P. Intramolecular addition of the riboflavin side chain. Anion-catalyzed neutral photochemistry. Eur J Biochem. 1975;57:35–48.
Ahmad I, Fasihullah Q, Vaid FHM. A study of simultaneous photolysis and photoaddition reactions of riboflavin in aqueous solution. J Photochem Photobiol B Biol. 2004;75:13–20.
Ahmad I, Fasihullah Q, Vaid FHM. Effect of phosphate buffer on photodegradation reactions of riboflavin in aqueous solution. J Photochem Photobiol B Biol. 2005;78:229–34.
Ahmad I, Fasihullah Q, Vaid FHM. Effect of light intensity and wavelength on photodegradation reactions riboflavin in aqueous solution. J Photochem Photobiol B Biol. 2006;82:21–7.
Ahmad I, Vaid FHM. Photochemistry of flavins in aqueous and organic solvents. In: Silva E, Edwards AM, editors. Flavins photochemistry and photobiology. Cambridge: Royal Society of Chemistry; 2006. p. 13–40.
Ahmad I, Ahmed S, Sheraz MA, Vaid FHM, Ansari IA. Effect of divalent anions on photodegradation kinetics and pathways of riboflavin in aqueous solution. Int J Pharm. 2010;390:174–82.
Ahmad I, Ahmed S, Sheraz MA, Vaid FHM. Effect of borate buffer on the photolysis of riboflavin in aqueous solution. J Photochem Photobiol B Biol. 2008;93:82–7.
Ahmad I, Sheraz MA, Ahmed S, Hafeez SK, Mirza T, Aminuddin M. Stabilizing effect of citrate buffer on the photolysis of riboflavin in aqueous solution. Result Pharma Sci. 2011;1:11–5.
Hou JP, Poole JW. Lactam antibiotics: their physicochemical properties and biological activities in relation to structure. J Pharm Sci. 1971;60:503–32.
Wood RW. Carbenicillin monograph. In: Connors CA, Amidon GL, Stella VJ, editors. Chemical stability of pharmaceuticals: a handbook for pharmacist. 2nd ed. New York: Wiley; 1986. p. 290–4.
Fabre H, Eddine NH, Berge G. Degradation kinetics in aqueous solution of cefotaxime sodium, a third-generation cephalosporin. J Pharm Sci. 1984;73:611–8.
Hajratwala BR. Kinetics of sulfite-induced anaerobic degradation of epinephrine. J Pharm Sci. 1975;64:45–8.
Hansen J, Kreilgard B, Nielsen, Veje J. Kinetics of degradation of methotrexate in aqueous solution. Int J Pharm. 1983;16:141–52.
Underberg WJM, Lingeman H. Aspects of the chemical stability of mitomycin and profiromycin in acidic solution. J Pharm Sci. 1983;72:549–53.
Stehle RG, Smith RW. Relative’s aqueous stabilities of dinoprostone free acid (prostaglandin E2) and its carbamomylmethyl ester. J Pharm Sci. 1976;65:1844–5.
Windheuser JJ, Higuchi T. Kinetics of thiamine hydrolysis. J Pharm Sci. 1962;51:354–64.
Pertzer D. Cis-platin monograph. In: Connors CA, Amidon GL, Stella VJ, editors. Chemical stability of pharmaceuticals: a handbook for pharmacist. 2nd ed. New York: Wiley; 1986. p. 356–64.
Reid JM. Cyclophosphamide monograph. In: Connors CA, Amidon GL, Stella VJ, editors. Chemical stability of pharmaceuticals: a handbook for pharmacist. 2nd ed. New York: Wiley; 1986. p. 385–93.
Konorev EA, Zhang A, Joseph J, Kennedy MC, Kalyanaraman B. Bicarbonate exacerbates oxidative injury induced by antitumor antibiotic doxorubicin in cardiomyocytes. AJP-Heart. 2000;279:2424–30.
Fall HH, Petering HG. Metabolic inhibitors.1. 6,7-dimethyl-9-formylmethylisoalloxazine, 6,7-dimethyl-9-(12-hydroxyethyl)-isoalloxazine and derivatives. J Am Chem Soc. 1956;78:377–81.
Fukumachi C, Sakurai Y. Vitamin B2 photolysis. V. The photolytic formation of 6, 7-dimethylflavin-9-acetic acid ester from riboflavin. Vitamins (Kyoto). 1954;7:939–43.
Ahmad I, Rapson HDC, Heelis PF, Phillips GO. Alkaline hydrolysis of 7, 8-dimethyl-10(formylmethyl)-isoalloxazine. A kinetic study. J Org Chem. 1980;45:31–3.
Hatchard CG, Parker CA. A new sensitive chemical actinometer. II. Potassium ferrioxalate as a standard chemical antinometer. Proc Roy Soc (Lond). 1956;A 235:518–36.
Ahmad I, Rapson HDC. Multicomponent spectrophotometeric assay of riboflavin and photoproducts. J Pharm Biomed Anal. 1990;8:217–23.
Suelter CH, Metzler DE. The oxidation of a reduced pyridine nucleotide analog by flavins. Biochem Biophys Acta. 1960;44:22–3.
Ahmad I, Fasihullah Q, Vaid FHM. Photolysis of formylmethylflavin in aqueous and organic solvents. Photochem Photobiol Sci. 2006;5:680–5.
Ahmad I, Mirza T, Iqbal K, Ahmed S, Sheraz MA, Vaid FHM. Effect of pH, buffer and viscosity on the photolysis of formylmethylflavin: a kinetic study. Aust J Chem. 2013;66:579–85.
Song PS, Metzler DE. Photochemical degradation of flavins. IV. Studies of the anaerobic photolysis of riboflavin. Photochem Photobiol. 1967;6:691–709.
Farrer KTH, Macewan JL. The thermal destruction of riboflavin. Aust J Biol Sci. 1954;7:73–84.
Harkness DR, Stadman ER. Bacterial degradation of riboflavin. J Biol Chem. 1965;240:4089–96.
Ahmad I, Ahmed S, Sheraz MA, Aminuddin M, Vaid FHM. Effect of caffeine complexation on the photolysis of riboflavin in aqueous solution: a kinetic study. Chem Pharm Bull. 2009;57:1363–70.
Cairns WL, Metzler DE. Photochemical degradation of flavins. VI. A new photoproduct and its use in studying the photolytic mechanism. J Am Chem Soc. 1971;93:2772–7.
Heelis PF, Phillips GO, Ahmad I, Rapson HDC. The photodegradation of formylmethylflavin—a steady state and laser flash photolysis study. Photochem Photobiophys. 1980;1:125–30.
Rodiguin NM, Rodiguina EN. Consecutive chemical reactions. Princelin: Van Nostand Co., Inc; 1964. p. 84–96.
O’Neil MJ, editor. The Merck Index. 15th ed. Cambridge, UK: The Royal Society of Chemistry; 2013. Electronic Version.
Moore WM, Spence JT, Raymond FA, Colson SD. Photochemistry of riboflavin. I. The hydrogen transfer process in the anaerobic photobleaching of flavins. J Am Chem Soc. 1963;85:3367–72.
Smith EC, Metzler DE. The photochemical degradation of riboflavin. J Am Chem Soc. 1963;85:3285–8.
Clark WM. Oxidation-reduction potentials of organic systems. Baltimore: Williams & Wilkins; 1960. p. 442.
Mayhew SG. The effects of pH and semiquinone formation on the oxidation-reduction potentials of flavin mononucleotide. A reappraisal. Eur J Biochem. 1999;265:698–702.
Wells JI. Pharmaceutical formulation: the physicochemical properties of drug substances. New York: Wiley; 1988. p. 171.
Albert A, Serjent EP. Ionization constants of acids and bases. A laboratory mannual. London: Methuen & Co; 1962. p. 151.
Weber G. Fluorescence of riboflavin and flavin adenine dinucleotide. Biochem J. 1950;47:114–21.
Heelis PF. The photophysical and photochemical properties of flavins (isoalloxazines). Chem Soc Rev. 1982;11:15–39.
Drossler P, Holzer W, Penzkoffer A, Hegemann P. Fluorescence quenching of aqueous solutions of riboflavin by methionin and cystein. Chim Phys. 2003;286:409.
Song PS. Chemistry of flavins in their excited states. In: Kamin H, editor. Flavins and flavoproteins. Baltimore: University Park Press; 1971. p. 37–61.
Heelis PF. The photochemistry of flavins. In: Muller F, editor. Chemistry and biochemistry of flavoenzymes, vol. 1. Boca Raton, FL: CRC Press; 1991. p. 171–93.
Gladys M, Knappe WR. Photochemie des (Iso) alloxazins III. Intramolekulare photodealkylierung von 10-alkylisoalloxazinen, eine mofellreaktion fur den riboflavin-photoabbau. Chem Ber. 1974;107:3658–73.
Knappe WR. Photochemie des (Iso) Alloxazins IV. Dealkylierung und decarboxylierung kurzkettiger isoalloxazin-10-alkancarbonsauren. Chem Ber. 1975;108:2422–38.
Sheraz MA, Kazi SH, Ahmad S, Mirza T, Ahmad I, Evstigneev MP. Effect of phosphate buffer on the complexation and photochemical interaction of riboflavin and caffeine in aqueous solution: a kinetic study. J Photochem Photobiol A Chem. 2014;273:17–22.
Ahmad I, Tollin G. Flavin triplet quenching and semiquinone formation by aliphatic α-substituted acetic acids: intermediates in flavin-sensitized photodecarboxylation. Photochem Photobiol. 1981;34:441–5.
Ahmad I, Tollin G. Solvent effects on flavin electron transfer reactions. Biochemistry. 1981;20:5925–8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahmad, I., Anwar, Z., Iqbal, K. et al. Effect of Acetate and Carbonate Buffers on the Photolysis of Riboflavin in Aqueous Solution: A Kinetic Study. AAPS PharmSciTech 15, 550–559 (2014). https://doi.org/10.1208/s12249-013-0067-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-013-0067-6