Abstract
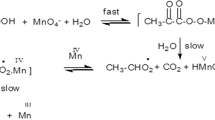
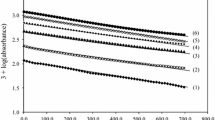
The kinetics of oxidation of riboflavin (RFH) by sodium metaperiodate (IO4 −) in aqueous acidic medium have been studied. The reaction showed first-order dependence on both reactants and inverse dependence on [H+] over the pH range 1.4–2.6. The deprotonated form of riboflavin was found to be more reactive than its conjugate acid, (RFH2 +). The polymerization of acrylonitrile provided evidence for an inner-sphere mechanism involving iodine(VI) free radicals. The main oxidation products were identified by TLC and mass spectra as lumichrome and 1-acetylglycerol. The effect of iron(II) on the rate of oxidation was studied over the range (0.96–6.0) × 10−6 mol dm−3, and the rate was found to decrease with [Fe2+] over the range studied.




Similar content being viewed by others
References
Josian M, Tolot C, Crisiti DB, Elias ZA, Santos ML (2005) Anal Chim Acta 531:279
Slyke DDV, Hiller A, Macfadyen DA, Hastings AB, Kemperer W (1994) J Biol Chem 133:287
Sulfab Y (1976) J Inorg Nucl Chem 38:2271
Sulfab Y, Abu-Shadi AI (1977) Inorg Chim Acta 21:115
Kasim AY, Sulfab Y (1977) Inorg Chem Acta 24:247
El-Eziri FR, Sulfab Y (1977) Inorg Chem Acta 25:15
Buist GJ (1972) In: Boneford CH, Tripper CFH (eds) Comprehensive chemical kinetics, vol 6. Elsevier, Amsterdam, p 435
Indelli A, Ferranti F, Secco F (1966) J Phys Chem 70:631
Symons MCR (1955) J Chem Soc. doi:10.1039/jr9550002794
Hussein MA, Sulfab Y (1981) Transition Met Chem 7:181
Garapati S, Parvataneni V (2010) RJPBCS 1:977
Abdel-Khalek AA, Khalil MM, Khalid IS (1993) Transition Met Chem 18:153
Rao BD, Sridevi M, Vani P (2013) Indian J Appl Res 3:585
Padavathil HT, Mavalangi S, Nandibewoor ST (2013) AIJRSTEM 3:63
Rajeshwari VH, Kirthi SB, Sharanappa TN, Shivamurti AC (2010) Z Phys Chem 225:79
Drossler P, Holzer W, Penzkofer A, Hagamann P (2003) Chem Phys 286:409
Treadwell GE, Cairns WL, Metzler DE (1968) J Chromatogr 35:376
Schunan JN, Schollnhammer G, Hemmerich P (1975) Eur J Biochem 57:35
Ahmed I, Fasihullah Q, Noor A, Ansari IA, Ali QNN (2004) Int J Farm 280:199
Weaver MJ, Yee EL (1980) Inorg Chem 19:1936
Kustin K, Lieberman EC (1964) J Phys Chem 68:3869
Kasim AY, Sulfab Y (1997) Inorg Chem Acta 22:169
Bridgart GI, Fuller MW, Wilson IR (1973) J Chem Soc Dalton Trans 1274.doi:10.1039/DT9730001274
Hadinecm I, Jenšovský L, Linek A, Syneček V (1960) Naturwiss Enschaften 47:377
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdel-Hady, A.E.M., Taha, A.M. Kinetics and mechanism of oxidation of riboflavin by periodate in aqueous acidic medium: evidence for the inhibiting effect of iron(II). Transition Met Chem 40, 379–385 (2015). https://doi.org/10.1007/s11243-015-9927-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-015-9927-0