Abstract
This study aims to investigate the application of impedance spectroscopy using fixed electrode geometries on a standard glass vial in the characterisation of the freezing process of solutions at different fill liquid volumes. Impedance spectra (between 10 and 106 Hz) were recorded every 3 min, during the freezing cycle on a solution of 30 mg/mL sucrose contained within 10 mL glass vials having an electrode system (two thin copper foils: w, 18 mm; h, 5 mm) affixed to the external surface of the vial. A fill factor (Φ) was defined in terms of the relative height of the solution volume to the height of the electrodes from the base of the vial. Solution volumes of 1.5 to 5 mL (corresponding to Φ= 0.5–1.6) were investigated to establish the applicability of having a fixed electrode geometry for a range of solution volumes. A linear relationship between the time duration of the ice formation/solidification phase and the fill factor suggests that fixed electrode geometries may be used to investigate a range of fill volumes. The benefit of this approach is that it does not invade the solution and hence records the freezing process without providing additional nucleation sites and in a manner which is representative of the entire fill volume.






Similar content being viewed by others
REFERENCES
Pikal MJ. Freeze drying. In: Swarbrick J, editor. Encyclopedia of pharmaceutical technology. 3rd ed. New York: Marcel Dekker; 2002. p. 1807–33.
James A, Searles JFC, Randolph TW. The ice nucleation temperature determines the primary drying rate of lyophilization for samples frozen on a temperature-controlled shelf. J Pharm Sci. 2001;90(7):860–71.
Hottot A, Vessot S, Andrieu J. Freeze drying of pharmaceuticals in vials: influence of freezing protocol and sample configuration on ice morphology and freeze-dried cake texture. Chem Eng Process. 2007;46(7):666–74.
Wilson PW, Heneghan AF, Haymet ADJ. Ice nucleation in nature: supercooling point (SCP) measurements and the role of heterogeneous nucleation. Cryobiology. 2003;46(1):88–98.
Rambhatla S, Ramot R, Bhugra C, Pikal M. Heat and mass transfer scale-up issues during freeze drying: II. Control and characterization of the degree of supercooling. AAPS PharmSciTech. 2004;5(4):54–62.
Liu J, Viverette T, Virgin M, Anderson M, Dalal P. A study of the impact of freezing on the lyophilization of a concentrated formulation with a high fill depth. Pharm Dev Technol. 2005;10(2):261–72.
Guttzeit M. Designing an effective PAT-driven scale-up of lyophilization processes. PharmTechnol. 2010;22(11):8.
Oetjen G-W. Foundations and process engineering. Freeze-drying. Weinheim: Wiley; 2007. p. 1–126.
Mousavi R, Miri T, Cox PW, Fryer PJ. A novel technique for ice crystal visualization in frozen solids using X-ray micro-computed tomography. J Food Sci. 2005;70(7):e437–42.
Ward KR, Matejtschuk P. The use of microscopy, thermal analysis, and impedance measurements to establish critical formulation parameters for freeze-drying cycle development. In: Rey L, May JC, editors. Freeze drying/lyophilization of pharmaceutical and biological products. New York: Marcel Dekker; 2010. p. 112–35.
Kasper JC, Friess W. The freezing step in lyophilization: physico-chemical fundamentals, freezing methods and consequences on process performance and quality attributes of biopharmaceuticals. Eur J Pharm Biopharm. 2011;78(2):248–63.
Konstantinidis AK, Kuu W, Otten L, Nail SL, Sever RR. Controlled nucleation in freeze-drying: effects on pore size in the dried product layer, mass transfer resistance, and primary drying rate. J Pharm Sci. 2011;100(8):3453–70.
Franks F. Freeze-drying of pharmaceuticals and biopharmaceuticals. Cambridge: The Royal Society of Chemistry; 2007.
Brülls M, Folestad S, Sparén A, Rasmuson A. Near-infrared spectroscopy monitoring of the lyophilization process. Pharm Res. 2003;20(3):494–9.
Mujat M, Greco K, Galbally-Kinney KL, Hammer DX, Ferguson RD, Iftimia N, et al. Optical coherence tomography-based freeze-drying microscopy. Biomed Opt Express. 2012;3(1):55–63.
De Beer TRM, Vercruysse P, Burggraeve A, Quinten T, Ouyang J, Zhang X, et al. In-line and real-time process monitoring of a freeze drying process using Raman and NIR spectroscopy as complementary process analytical technology (PAT) tools. J Pharm Sci. 2009;98(9):3430–46.
Macdonald JR, Johnson WB. Fundamentals of impedance spectroscopy. In: Barsoukov E, Macdonald JR, editors. Impedance spectroscopy theory, experiment and applications. 2nd ed. New Jersey: Wiley; 2005. p. 595.
Suherman PM, Smith G. A percolation cluster model of the temperature dependent dielectric properties of hydrated proteins. Journal of Physics D-Applied Physics. 2003;36(4):336–42.
Petrovsky V, Jasinski P, Dogan F. Effective dielectric constant of two phase systems: application to mixed conducting systems. J Appl Phys. 2012;112(3):034107.
Ermolina I, Smith G. Dielectric spectroscopy of low-losses sugar lyophiles: III: the influence of moisture on the dielectric response of freeze-dried lactose. J Non-Cryst Solids. 2011;357(2):671–6.
Soley A, Lecina M, Gámez X, Cairó JJ, Riu P, Rosell X, et al. On-line monitoring of yeast cell growth by impedance spectroscopy. J Biotechnol. 2005;118(4):398–405.
Olmi R, Meriakri VV, Ignesti A, Priori S, Riminesi C. Monitoring alcoholic fermentation by microwave dielectric spectroscopy. J Microw Power Electromagn Energy. 2007;41(3):37–49. Epub 2008/03/21.
Smith G, Polygalov E, Page T, inventors; GEA Pharma Systems Limited, assignee. Electrical monitoring of a lyophilization process Great Britain patent GB2480299. 2011; 16/11/2011.
Smith G, Polygalov E, Arshad MS, Page T, Taylor J, Ermolina I. An impedance-based process analytical technology for monitoring the lyophilisation process. Int J Pharm. 2013;449(1–2):72–83.
Smith G, Arshad MS, Polygalov E, Ermolina I. An application for impedance spectroscopy in the characterisation of the glass transition during the lyophilization cycle: the example of a 10% w/v maltodextrin solution. Eur J Pharm Biopharm. 2013. doi:10.1016/j.ejpb.2013.08.004.
Nakagawa K, Hottot A, Vessot S, Andrieu J. Modeling of freezing step during freeze-drying of drugs in vials. AIChE J. 2007;53(5):1362–72.
ACKNOWLEDGMENTS
The current impedance measurement system (LyoDEA™) was developed through collaboration with GEA Pharma Systems, AstraZeneca, and Ametek, and co-funded by the Technology Strategy Board.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendices
Appendix 1: Estimation of the Peak Frequencies of Liquid and Frozen Sucrose Solution (30 mg/mL) Within Tubing Vial of Different Vial Geometries
In a simple approximation, the impedance of the object under test can be described as a combination of resistor and capacitor; the resistance of which is defined by following equation
where K 1 is a geometrical coefficient, d is the internal diameter of the vial, A CS is an area of effective vertical cross section of the sample (i.e. the solution within the vial) and σ is specific conductivity of the sample. The capacitance can be defined by the following equation.
Where ε 0 is the permittivity of a vacuum, ε is the dielectric constant of glass, A is the area of the electrodes and l is thickness of the glass wall. For the purpose of these calculations, the wall thickness is assumed to be constant for all sizes of vial.
Multiplying R by C, we obtain
where τ is the known as the time constant of the serial RC circuit. It is this time constant which defines the position of the interfacial relaxation peak in the experimental frequency window (where f peak = 1/2πτ).
In the first approximation, A CS can be presented as A CS = AK 2 where K 2 is a constant coefficient (associated with the fixed cylindrical shape of the sample volume). Then Eq. (3) can be rewritten as
A in the numerator and denominator can be cancelled thus giving
For simplicity, let us denote
As all members in the right side of Eq. (6) are constants then K p (the proportionality coefficient) is also a constant, and expression (5) can be simplified to
and respectively
Expression (8) shows that the frequency position of the peak has an approximately inverse dependence on the internal diameter of the vial.
Having measured the experimental value of f peak for 10 mL (f peak10 ml), it is then straight forward to estimate f peak(xml) for different sized vials from the ratio of the diameters, according to the formula below.
Table I gives theoretical estimates for the peak frequency for different sized vials, for both the liquid state and the frozen state.
Appendix 2: Thermal Mass Contributions from the External Electrodes
The basis for these calculations is to first determine the position of the top of the guard electrode from the base of the vial (dimension A; Fig. 7) for a fill volume that provides a constant ratio of the liquid cross sectional area to the liquid fill height, for all sizes of vial and which is equal to that for a 3-mL fill volume in a 10-mL vial.
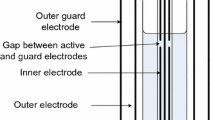
Schematic of the electrode assembly. A electrode height, B length of guard electrode, C height of stimulating/sensing electrode, D width of stimulating/sensing electrode, E width of guard electrode, F spacing between guard electrode and sensing electrode, and G is the height of the side segment of the guard electrode
Once the position of the top of the guard electrode is defined, then the next step is to estimate the length of the sensing/stimulating electrode (dimension D; Fig. 7). This dimension is defined by the ratio of the electrode length to the circumference of the vial, which is fixed at 0.4 for all vials. All other dimensions, i.e. the separation/gap between the guard and the sensing/stimulating electrodes and the width of the guard electrode are fixed at 1 and 1.5, respectively. Knowing the dimensions A, D, E and F permits the calculation of all other dimensions, from which the total area of the electrode assembly can then be calculated (Table II). The mass of the electrode assembly is then determined from the specific weight of the electrode material (0.4 mg/mm2 for the copper foil used on the 10 mL vial) and the %increase in vial weight from attaching the copper foil is then determined from the weight of the vial. Table III shows the dimensions of the electrode assemblies and the results of these calculations of %increase in mass.
Rights and permissions
About this article
Cite this article
Smith, G., Arshad, M.S., Polygalov, E. et al. Factors Affecting the Use of Impedance Spectroscopy in the Characterisation of the Freezing Stage of the Lyophilisation Process: the Impact of Liquid Fill Height in Relation to Electrode Geometry. AAPS PharmSciTech 15, 261–269 (2014). https://doi.org/10.1208/s12249-013-0054-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-013-0054-y