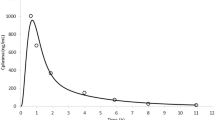
Abstract
Physiologically based pharmacokinetic (PBPK) modeling has become a useful tool to estimate the performance of orally administrated drugs. Here, we described multiple in silico/in vitro/in vivo tools to support formulation development toward mitigating the positive food effect of NVS123, a weak base with a pH-dependent and limited solubility. Administered orally with high-fat meal, NVS123 formulated as dry filled capsules displayed a positive food effects in humans. Three alternative formulations were developed and assessed in in vitro and in vivo preclinical and/or clinical studies. By integrating preclinical in vitro and in vivo data, the PBPK model successfully estimated the magnitude of food effects and the predicted values were within ±30% of the observed results. A model-guided parameter sensitivity analysis illustrated that enhanced solubility and longer precipitation times under fed condition were the main reason for enhanced NVS123's exposure in presence of food. Eventually, exposure after an amorphous formulation was found to be not significantly altered because of remarkably enhanced intestinal solubility and reduced precipitation. Gastroplus population simulations also suggested that the amorphous formulation is promising in mitigating a clinically significant food effect. Overall, these efforts supported the rationale of clinical investigation of the new formulation, and more importantly, highlighted a practical application of PBPK modeling solving issues of undesirable food effects in weakly basic compounds based on preclinical in vitro/in vivo data.






Similar content being viewed by others
References
Fleisher D, Li C, Zhou Y, Pao LH, Karim A. Drug, meal and formulation interactions influencing drug absorption after oral administration. Clinical implications. Clin Pharmacokinet. 1999;36(3):233–54.
Benet LZ. WCY. Using a biopharmaceutics drug disposition classification system to predict bioavailability and elimination characteristics of new molecular entities. Somerset, NJ: NJDMDG; 2006.
Custodio JM, Wu C-Y, Benet Leslie Z. Predicting drug disposition, absorption/elimination/transporter interplay and the role of food on drug absorption. Adv Drug Deliv Rev. 2008;60(6):717–33.
FDA. Food-Effect Bioavailability and Fed Bioequivalence Studies. Guidance for Industry 2002. Available from: http://www.fda.gov/downloads/regulatoryinformation/guidances/ucm126833.pdf. Accessed 02 Jun 2012
Wu CY, Benet LZ. Predicting drug disposition via application of BCS: transport/absorption/elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm Res. 2005;22(1):11–23.
Parrott N, Lukacova V, Fraczkiewicz G, Bolger MB. Predicting pharmacokinetics of drugs using physiologically based modeling-application to food effects. AAPS J. 2009;11(1):45–53.
Jones HM, Parrott N, Ohlenbusch G, Lave T. Predicting pharmacokinetic food effects using biorelevant solubility media and physiologically based modelling. Clin Pharmacokinet. 2006;45(12):1213–26.
Heimbach T, Xia B, Lin TH, He H. Case studies for practical food effect assessments across BCS/BDDCS class compounds using in silico, in vitro, and preclinical in vivo data. AAPS J. 2013;15(1):143–58.
Yu LX, Amidon GL. Characterization of small intestinal transit time distribution in humans. Int J Pharm. 1998;171(2):157–63.
Gardner JD, Ciociola AA, Robinson M. Measurement of meal-stimulated gastric acid secretion by in vivo gastric autotitration. J Appl Physiol. 2002;92(2):427–34.
Langenbucher F. Linearization of dissolution rate curves by the Weibull distribution. J Pharm Pharmacol. 1972;24(12):979–81.
Mithani SD, Bakatselou V, TenHoor CN, Dressman JB. Estimation of the increase in solubility of drugs as a function of bile salt concentration. Pharmaceut Res. 1996;13(1):163–7.
Lentz KA, Quitko M, Morgan DG, Grace Jr JE, Gleason C, Marathe PH. Development and validation of a preclinical food effect model. J Pharm Sci. 2007;96(2):459–72.
Lentz KA. Current methods for predicting human food effect. AAPS J. 2008;10(2):282–8.
Wang J, Flanagan DR. General solution for diffusion-controlled dissolution of spherical particles. 1. Theory. J Pharm Sci. 1999;88(7):731–8.
Zhang X, Lionberger RA, Davit BM, Yu LX. Utility of physiologically based absorption modeling in implementing quality by design in drug development. AAPS J. 2011;13(1):59–71.
Akimoto M, Nagahata N, Furuya A, Fukushima K, Higuchi S, Suwa T. Gastric pH profiles of beagle dogs and their use as an alternative to human testing. Eur J Pharm Biopharm. 2000;49(2):99–102.
Lui CY, Amidon GL, Berardi RR, Fleisher D, Youngberg C, Dressman JB. Comparison of gastrointestinal Ph in dogs and humans—implications on the use of the beagle dog as a model for oral absorption in humans. J Pharm Sci. 1986;75(3):271–4.
Meyer JH, Dressman J, Fink A, Amidon G. Effect of size and density on canine gastric-emptying of nondigestible solids. Gastroenterology. 1985;89(4):805–13.
Acknowledgments
The authors would like to thank Dr. Akash Jain and members of the Novartis Food Effect Quality Plus, preclinical PK/PD, clinical pharmacology, and oral formulation development team for conducting preclinical and clinical studies as well as general scientific discussions and inputs.
Conflict of Interests
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: Divyakant Desai, John Crison, and Peter Timmins
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 32 kb)
Rights and permissions
About this article
Cite this article
Xia, B., Heimbach, T., Lin, Th. et al. Utility of Physiologically Based Modeling and Preclinical In Vitro/In Vivo Data to Mitigate Positive Food Effect in a BCS Class 2 Compound. AAPS PharmSciTech 14, 1255–1266 (2013). https://doi.org/10.1208/s12249-013-0018-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-013-0018-2