Abstract
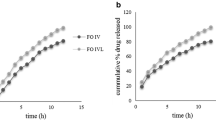
Interpolyelectrolyte (IPE) complexation between carrageenan (CG) and Eudragit E (EE) was studied in 0.1 M HCl and was used to develop floating matrix tablets aimed to prolong gastric-residence time and sustain delivery of the loaded drug. The optimum EE/CG IPE complexation weight ratio (0.6) was determined in 0.1 M HCl using apparent viscosity measurements. The IPE complex was characterized by Fourier transform infrared spectroscopy and differential scanning calorimetry. Metronidazole matrix tablets were prepared by direct compression using EE, CG, or hybrid EE/CG with ratio optimal for IPE complexation. Corresponding effervescent tablets were prepared by including Na bicarbonate as an effervescent agent. Tablets were evaluated for in vitro buoyancy and drug release in 0.1 M HCl. Both CG and EE–CG effervescent matrices (1:2 drug to polymer weight ratio, 60 mg Na bicarbonate) achieved fast and prolonged floating with floating lag times less than 30 s and floating duration of more than 10 h. The corresponding EE effervescent matrices showed delayed floating and rapid drug release, and completely dissolved after 3 h of dissolution. CG matrices showed an initial burst drug release (48.3 ± 5.0% at 1 h) followed by slow drug release over 8 h. EE–CG matrices exhibited sustained drug release in almost zero-order manner for 10 h (68.2 ± 6.6%). The dissolution data of these matrices were fitted to different dissolution models. It was found that drug release followed zero-order kinetics and was controlled by the superposition of the diffusion and erosion.








Similar content being viewed by others
REFERENCES
Risbud MV, Hardikar AA, Bhat SV, Bhonde RR. pH-sensitive freeze-dried chitosan-polyvinyl pyrrolidone hydrogels as controlled release system for antibiotic delivery. J Control Release. 2000;68:23–30.
Burton S, Washington N, Steele RJ, Musson R, Feely L. Intragastric distribution of ion-exchange resins: a drug delivery system for the topical treatment of the gastric mucosa. J Pharm Pharmacol. 1995;47:901–6.
Wu Y, Fassihi R. Stability of metronidazole, tetracycline hcl and famotidine alone and in combination. Int J Pharm. 2005;290:1–13.
Santus G, Lazzarini C, Bottoni G, Sandefer EP, Page RC, Doll WJ, et al. An in vitro-in vivo investigation of oral bioadhesive controlled release furosemide formulations. Eur J Pharm Biopharm. 1997;44:39–52.
Deshpande AA, Rhodes CT, Shah NH, Malick AW. Controlled-release drug delivery systems for prolonged gastric residence: an overview. Drug Dev Ind Pharm. 1996;22:531–9.
Deshpande AA, Shah NH, Rhodes CT, Malick AW. Development of a novel controlled-release system for gastric retention. Pharm Res. 1997;14:815–9.
Menon A, Ritschel WA, Sakr A. Development and evaluation of a monolithic floating dosage form for furosemide. J Pharm Sci. 1994;83:239–45.
Whitehead L, Fell JT, Collett JH, Sharma HL, Smith AM. Floating dosage forms: an in vivo study demonstrating prolonged gastric retention. J Control Release. 1998;55:3–12.
Gambhire MN, Ambade KW, Kurmi SD, Kadam VJ, Jadhav KR. Development and in vitro evaluation of an oral floating matrix tablet formulation of diltiazem hydrochloride. AAPS PharmSciTech. 2007;8:E73.
Li S, Lin S, Chien YW, Daggy BP, Mirchandani HL. Statistical optimization of gastric floating system for oral controlled delivery of calcium. AAPS PharmSciTech. 2001;2:E1.
Xu G, Groves MJ. Effect of FITC-dextran molecular weight on its release from floating cetyl alcohol and HPMC tablets. J Pharm Pharmacol. 2001;53:49–56.
Singh BN, Kim KH. Floating drug delivery systems: an approach to oral controlled drug delivery via gastric retention. J Control Release. 2000;63:235–59.
Shan-Yang L, Hui-Ling Y. Microscopic Fourier transform infrared/differential scanning calorimetry system used to study the different thermal behaviors of polymethacrylate copolymers of Eudragits RS, RL, E 30D, or E. J Appl Polym Sci. 2000;78:8298–35.
Shan-Yang L, Hui-Ling Y, Mei-Jane L. Formation of six-membered cyclic anhydrides by thermally induced intramolecular ester condensation in Eudragit E Film. Polymer. 1999;40:3589–93.
Picker K. The use of carrageenan in mixture with microcrystalline cellulose and its functionality for making tablets. Eur J Pharm Biopharm. 1999;48:27–36.
Prado HJ, Matulewicz MC, Bonelli P, Cukierman AL. Basic butylated methacrylate copolymer/kappa-carrageenan interpolyelectrolyte complex: preparation, characterization and drug release behaviour. Eur J Pharm Biopharm. 2008;70:171–8.
Jaimini M, Rana AC, Tanwar YS. Formulation and evaluation of famotidine floating tablets. Curr Drug Deliv. 2007;4:51–5.
Bakre LG, Jaiyeoba KT. Effects of drying methods on the physicochemical and compressional characteristics of okra powder and the release properties of its metronidazole tablet formulation. Arch Pharm Res. 2009;32:259–67.
Sankalia MG, Mashru RC, Sankalia JM, Sutariya VB. Stability improvement of alpha-amylase entrapped in kappa-carrageenan beads: physicochemical characterization and optimization using composite index. Int J Pharm. 2006;312:1–14.
Moustafine R, Zaharov I, Kemenova V. Physicochemical Characterization and Drug Release Properties of Eudragit E PO/Eudragit L 100-55 Inter Polyelectrolyte Complexes. Eur J Pharm Biopharm. 2006;63:26–36.
Bonferoni M, Rossi S, Ferrari F, Bettinetti G, Caramella C. Characterization of a diltiazem-λ-carrageenan complex. Int J Pharm. 2000;200:207–16.
Korsmeyer R, Gurny R, Doelker E, Buri P, Peppas N. Mechanism of solute release from porous hydrophilic polymers. Int J Pharm. 1983;15:25–35.
Peppas N. Analysis of Fickian and non-Fickian drug release from polymers. Pharm Acta Helv. 1985;60:110–1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bani-Jaber, A., Al-Aani, L., Alkhatib, H. et al. Prolonged Intragastric Drug Delivery Mediated by Eudragit® E-Carrageenan Polyelectrolyte Matrix Tablets . AAPS PharmSciTech 12, 354–361 (2011). https://doi.org/10.1208/s12249-011-9595-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-011-9595-0