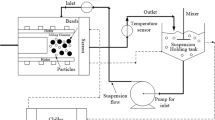
Abstract
Liquid mixing scale-up in pharmaceutical industry has often been based on empirical approach in spite of tremendous understanding of liquid mixing scale-up in engineering fields. In this work, we attempt to provide a model-based approach to scale-up dissolution process from a 2 l lab-scale vessel to a 4,000 l scale vessel used in manufacturing. Propylparaben was used as a model compound to verify the model predictions for operating conditions at commercial scale that would result in similar dissolution profile as observed in lab scale. Geometric similarity was maintained between both of the scales to ensure similar mixing characteristics. We utilized computational fluid dynamics (CFD) to ensure that the operating conditions at laboratory and commercial scale will result in similar power per unit volume (P/V). Utilizing this simple scale-up criterion of similar P/V across different scales, results obtained indicate fairly good reproducibility of the dissolution profiles between the two scales. Utilization of concepts of design of experiments enabled summarizing scale-up results in statistically meaningful parameters, for example −90% dissolution in lab scale at a given time under certain operating conditions will result in 75–88% at commercial scale with 95% confidence interval when P/V is maintained constant across the two scales. In this work, we have successfully demonstrated that scale-up of solid dissolution can be done using a systematic process of lab-scale experiments followed by simple CFD modeling to predict commercial-scale experimental conditions.







Similar content being viewed by others
Abbreviations
- a :
-
Interfacial area (m2/m3)
- k :
-
Interface solid–liquid mass transfer coefficient
- C :
-
Concentration of dissolved solid in bulk liquid (mol/m3)
- C*:
-
Concentration of dissolved solid at the interface (mol/m3)
- D :
-
Impeller diameter (m)
- N D :
-
Molecular diffusivity (m2/s)
- K :
-
Mass transfer coefficient (m/s)
- Rep :
-
Particle Reynolds number \((= {{{{\rho_{\rm{f}}}{\varepsilon^{{{{1} \left/ {3} \right.}}}}d_{\rm{p}}^{{{{4} \left/ {3} \right.}}}}} \left/ {{{\mu_{\rm{f}}}}} \right.} )\)
- Sc:
-
Schmidt number (=ϑ/D)
- Sh:
-
Sherwood number (=k d p/D)
- T :
-
Tank diameter (m)
- u t :
-
Terminal settling velocity of particle (m/s)
- N :
-
Impeller speed (rev/s)
- N SG :
-
Minimum impeller speed for complete suspension of the particles
- ρ f :
-
Fluid density (kg/m3)
- ε :
-
Turbulent dissipation rate (m2/s3)
- μ f :
-
Absolute viscosity (N.m/s)
- ϑ :
-
Kinematic viscosity (m2/s)
References
Brian PLT, Hales HB, Sherwood TK. Transport of heat and mass between liquids and spherical particles in an agitated tank. AIChE J. 1969;15:727–33.
Levins DM, Glastonbury JR. Application of Kolmogorofff’s theory to particle–liquid mass transfer in agitated vessels. Chem Eng Sci. 1972;27:537–43.
Levins DM, Glastonbury JR. Particle-liquid hydrodynamics and mass transfer in a stirred vessel. 2. Mass transfer. Trans Inst Chem Eng. 1972;50:132–46.
Okamoto Y, Nishikawa M, Hashimoto K. Energy Dissipation rate distribution in mixinig vessels and its effects on liquid–liquid dispersion and solid-liquid mass transfer. Int Chem Eng. 1981;21:88–91.
Kresta SM, Paul EL. Handbook of Industrial Mixing: Science and Practice. New York: Wiley; 2003.
Pangarkar VG, Yawalkar AA, Sharma MM, Beenackers AACM. Particle–liquid mass transfer coefficient in two-/three-phase stirred tank reactors. Ind Eng Chem Res. 2002;41:4141–67.
Perry RH, Green DW. Perry’s Chemical Engineers’ Handbook. New York: McGrawHill; 2008.
Barker JJ, Treybal RE. Mass-transfer coefficients for solids suspended in agitated liquids. AIChE J. 1960;6:289–95.
Hughmark GA. Hydrodynamics and mass transfer for suspended solid particles in a turbulent liquid. AIChE J. 1974;20:202–4.
Yu Lawrence X. X. Pharmaceutical quality by design: product and process development, understanding, and control. Pharm Res. 2008;25:781–91.
Ranade VV. Computational flow modeling for chemical reactor engineering. San Diego, CA: Academic Press; 2002.
Yeoh SL, Papadakis G, Yianneskis M. Numerical simulation of turbulent flow characteristics in a stirred vessel using the LES and RANS approaches with the sliding/deforming mesh methodology. Chem Eng Res Des. 2004;82:834–48.
Rielly CD, Habib M, Sherlock JP. Flow and mixing characteristics of a retreat curve impeller in a conical-based vessel. Chem Eng Res Des. 2007;85:953–62.
Jaworski Z, Bujalski W, Otomo N, Nienow AW. CFD study of homogenization with dual Rushton turbines. Comparison with experimental results. Part I: initial studies. Chem Eng Res Des. 2000;78:327–33.
Grant DJW, Mehdizadeh M, Chow AHL, Fairbrother JE. Non-linear van’t Hoff solubility-temperature plots and their pharmaceutical interpretation. Int J Pharm. 1984;18:25–38.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koganti, V., Carroll, F., Ferraina, R. et al. Application of Modeling to Scale-up Dissolution in Pharmaceutical Manufacturing. AAPS PharmSciTech 11, 1541–1548 (2010). https://doi.org/10.1208/s12249-010-9533-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-010-9533-6