Abstract
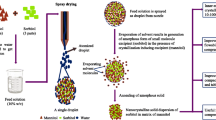
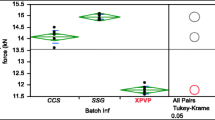
A co-processed excipient was prepared from commercially available crystalline mannitol and α-chitin using direct compression as well as spray, wet, and dry granulation. The effect of the ratio of the two components, percentage of lubricant and particle size, on the properties of the prepared co-processed excipient has been investigated. α-Chitin forms non-hygroscopic, highly compactable, disintegrable compacts when co-processed with crystalline mannitol. The compaction properties of the co-processed mannitol–chitin mixture were found to be dependent upon the quantity of mannitol added to chitin, in addition to the granulation procedure used. Optimal physicochemical properties of the excipient, from a manufacturing perspective, were obtained using a co-processed mannitol–chitin (2:8, w/w) mixture prepared by wet granulation (Cop-MC). Disintegration time, crushing strength, and friability of tablets, produced from Cop-MC using magnesium stearate as a lubricant, were found to be independent of the particle size of the prepared granules. The inherent binding and disintegration properties of the compressed Cop-MC are useful for the formulation of poorly compressible, high-strength, and low-strength active pharmaceutical ingredients. The ability to co-process α-chitin with crystalline mannitol allows chitin to be used as a valuable industrial pharmaceutical excipient.











Similar content being viewed by others
References
Peck GE, Baley GJ, McCurdy VE, Banker GS. Tablet formulation and design. In: Lieberman HA, Lachman L, Schwartz JB, editors. Pharmaceutical dosage forms: tablets, vol. 1. 2nd ed. New York: Marcel Dekker; 1989. p. 88–9.
Block LH, Moreton RC, Apte SP, Wendt RH, Munson EJ, Creekmore JR, et al. Co-processed excipients. In: Pharmacopeial forum, vol. 35 (4). Maryland, USA: United States Pharmacopeia Convention Inc;2009. p. 1026–8.
Westerhuis JA, de Haan P, Zwinkels J, Jansen WT, Coenegracht PJM, Lerk CF. Optimisation of the composition and production of mannitol/microcrystalline cellulose tablets. Int J Pharm. 1996;143:151–62.
Jian-Xin LI, Brian C, Thomas R. Co-processed microcrystalline cellulose and sugar alcohol as an excipient for tablet formulations. Applicant: FMC Corp. (US), European Patent Office, Patent No. US20080131505.
Sherwood BE, Hunter EA, Staniforth JH. Pharmaceutical excipient having improved compressibility. Applicant: Edward H Mendell Co. Inc. (US), United States Patent Office, Patent No. 5,585,115.
Muzzarelli RAA. Chitin. Oxford: Pergamon; 1977.
Safety data for chitin. http://msds.chem.ox.ac.uk/CH/chitin.html. Access date: 1/7/2010.
Uragami T, Tokura S. Material science of chitin and chitosan. Berlin: Springer; 2006.
Mir VG, Heinämäki J, Antikainen O, Revoredo OB, Colarte AI, Nieto OM, et al. Direct compression properties of chitin and chitosan. Eur J Pharm Biopharm. 2008;69:964–8.
Rashid I, Daraghmeh N, Al-Remawai M, Leharne SA, Chowdhry BZ, Badwan A. Characterization of chitin–metal silicates as binding superdisintegrants. J Pharm Sci. 2009;98:4887–901.
Rashid I, Al-Remawi M, Eftaiha A, Badwan A. Chitin–silicon dioxide coprecipitate as a novel superdisintegrant. J Pharm Sci. 2008;97:4955–69.
El-Barghouthi M, Rashid I, Eftaiha A, Al-Remawi M, Badwan A. A novel superdisintegrating agent made from physically modified chitosan with silicon dioxide. Drug Dev Ind Pharm. 2008;34:373–83.
Armstrong NA. Mannitol. In: Rowe RC, Sheskey PJ, Owen SC, editors. Pharmaceutical excipients. USA: Pharmaceutical Press and American Pharmacists Association; 2006. p. 439–53.
Erik L, Philippe L, Jose L. Pulverulent mannitol and process for preparing it. Applicant: Roquette Freres, Erik L, Philippe L, Jose L, United States Patent Office, Patent No. 6,743,447.
Daraghmeh NH, Al Omari MM, Badwan AA. Pharmaceutical excipient, method for its preparation and use thereof. European Patent Office (EPO). Patent Filed.
Gupta P, Nachaegari SK, Bansal AK. Improved excipient functionality by coprocessing. In: Katdsre A, Chaubal MV, editors. Excipient development for pharmaceutical, biotechnology, and drug delivery systems. New York: Informa Healthcare; 2006. p. 123.
Disintegration, friability of uncoated tablets, resistance to crushing of tablets. In: British pharmacopeia. London: The Stationary Office. Volume IV, Appendixes XIIA, XVII G and H; 2008. p. A283, A423, A424.
Weast RC. Handbook of chemistry and physics. 55th ed. Boca Raton: CRC; 1974–1975.
Dissolution <711>. United States Pharmacopeia and National Formulary (USP32-NF27). Rockville, MD: US Pharmacopoeia Convention. Volume 1;2009. p. 263–71.
Dissolution method. U.S. FDA, Rockville. 2010. http://www.fda.gov/scripts/cder/dissolution/index.cfm. Access date 4 Feb 2010.
Kawakita K, Ludde KH. Some considerations on powder compression equations. Powder Technol. 1971;4:61–8.
Shivanand P, Sprockel OL. Compaction behaviour of cellulose polymers. Powder Technol. 1992;69:177–84.
Lin C, Cham T. Compression behaviour and tensile strength of heat-treated polyethylene glycols. Int J Pharm. 1995;118:169–79.
Nordström J, Klevan I, Alderborn G. A particle rearrangement index based on the Kawakita powder compression equation. J Pharm Sci. 2008;98:1053–63.
Giron D. Applications of thermal analysis in the pharmaceutical industry. J Pharm Biomed Anal. 1986;4:755–70.
Botha SA, Lotter AP. Compatibility study between naproxen and tablet excipients using differential scanning calorimetry. Drug Dev Ind Pharm. 1990;16:673–83.
Lin SY, Han RY. Differential scanning calorimetry as a screening technique to determine the compatibility of salbutamol sulfate with excipients. Pharmazie. 1992;47:266–8.
Badwan AA. The Jordanian Pharmaceutical Manufacturing Co., Unpublished data.
Validation of compendia procedures <1225>. In: United States Pharmacopeia and National Formulary (USP32-NF27). Rockville, MD: US Pharmacopoeia Convention. Volume I;2009. p. 733–6.
Methyldopa tablets. In: United States Pharmacopeia and National Formulary (USP32-NF27). 2009. Rockville, MD: US Pharmacopoeia Convention. Volume 3;2009. p. 2942.
Qiang D, Gunn J, Zong Z, Buckner I. Evaluating the effect of lubrication on powder compaction with the compression calorimeter. AAPS Journal. 2009;11(S2). http://www.aapsj.org/abstracts/AM_2009/AAPS2009-001716.PDF. Accessed 2 March 2010.
Carlson GT, Hancock BC. A comparison of physical and chemical properties of common tableting diluents. In: Katdsre A, Chaubal MV, editors. Excipient development for pharmaceutical, biotechnology, and drug delivery systems. New York: Informa Healthcare; 2006. p. 129.
Nyström C, Alderborn G, Duberg M, Karehill P-G. Bonding surface area and bonding mechanism—two important factors for the understanding of powder compactibility. Drug Dev Ind Pharm. 1993;19:2143–96.
Alderborn G, Börjesson E, Glazer M, Nyström C. Studies on direct compression of tablets. XIX. The effect of particle size and shape on the mechanical strength of sodium bicarbonate tablets. Acta Pharm Suec. 1988;25:31–40.
Paronen P, Iilla J. Porosity–pressure functions. In: Alderborn G, Nyström C, editors. Pharmaceutical powder compaction technology. New York: Marcel Dekker; 1996. p. 55–75.
Adetunji OA, Odeniyi MA, Itiola OA. Compression, mechanical and release properties of chloroquine phosphate tablets containing corn and trifoliate yam starches as binders. Trop J Pharm Res. 2006;5:589–96.
Zhang Y, Law Y, Chakrabarti S. Physical properties and compact analysis of commonly used direct compression binders. AAPSPharmSciTech 2005;4(4), Article 62.
Summary of product characteristics (SPC) for Crestor® 20 and Istin® 10 tablets. In: Electronic Medicines Compendium. 2009 edition, Datapharm Communications Ltd, Leatherhead, United Kingdom. http://emc.medicines.org.uk/default.aspx. Accessed 2 March 2010.
Richard CJ, Alfred WN. Pharmaceutical composition comprising A HMG COA reductase inhibitor. Applicant: Astrazeneca AB (SE), European Patent Office. Patent No. EP1223918.
Fritz B, Adriaan DSP, Martin S. Crystalline forms of rosuvastatin calcium salt. Applicants: Ciba SC Holding AG, Fritz B, Adriaan DSP, Martin S, European Patent Office, Patent No. WO2006079611.
Shlomit W, Valerie N-H, Shalom S. Crystalline rosuvastatin calcium. Applicant: Teva Pharma, Shlomit W, Valerie N-H, Shalom S, European Patent Office, Patent No. WO2008036286.
Koichi W, Hikaru F. Tablet formulation. Applicant: NOVO NORDISK AS. (DK), European Patent Office, Patent No. US2009252790.
The Internet Drug Index (RxList). 2010. http://www.rxlist.com/aldomet-drug.htm. Accessed 4 Feb 2010.
Abdoh A, Al-Omari MM, Badwan AA, Jaber AMY. Amlodipine besylate–excipients interaction in solid dosage form. Pharmaceut Dev Tech. 2004;9:15–24.
Agatonovic-Kustrin S, Markovic N, Ginic-Markovic M, Mangan M, Glass BD. Compatibility studies between mannitol and omeprazole sodium isomers. J Pharm Biomed Anal. 2008;48:356–60.
ICH Topic Q1 A (R2) Stability testing of new drug substances and products. European Medicine Agency (EMEA). 2003. http://www.ema.europa.eu/pdfs/human/ich/273699en.pdf. Accessed 4 Feb 2010.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: Otilia Koo, Thomas Farrell, Allison Radwick, and Sameer Late
Rights and permissions
About this article
Cite this article
Daraghmeh, N., Rashid, I., Al Omari, M.M.H. et al. Preparation and Characterization of a Novel Co-processed Excipient of Chitin and Crystalline Mannitol. AAPS PharmSciTech 11, 1558–1571 (2010). https://doi.org/10.1208/s12249-010-9523-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-010-9523-8