Abstract
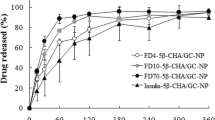
The aim of this paper was to evaluate the penetration enhancement properties of nanoparticles (NP) based on N-trimethyl chitosan (TMC 35% quaternization degree) loaded with insulin. The permeation performances of TMC NP were compared with those of chitosan (CS) NP and also with TMC and CS solutions. To estimate the mechanism of penetration enhancement, two different approaches have been taken into account: an in vitro study (Caco-2 cells) and an ex vivo study (excised rat duodenum, jejunum, and ileum). Insulin-loaded CS and TMC NP had dimensions of about 250 nm and had high yield and high encapsulation efficiency. The in vitro study evidenced that TMC and CS were able to enhance insulin permeation to the same extent. Penetration enhancement properties of TMC NP seem to be prevalently related to endocytosis while the widening of tight junctions appeared more important as mechanism in the case of CS NP. The ex vivo study put in evidence the role of mucus layer and of its microclimate pH. In duodenum (pH 5–5.5), CS and TMC solutions were more effective than NP while TMC NP were more efficient towards jejunum tissue (pH 6–6.5) for their high mucoadhesive potential. Confocal laser scanning microscopy study supported the hypothesis that penetration enhancement due to TMC NP was mainly due to internalization/endocytosis into duodenum and jejunum epithelial cells. The good penetration enhancement properties (permeation and penetration/internalization) make TMC NP suitable carriers for oral administration of insulin.









Similar content being viewed by others
References
Aboubakar M, Couvreur P, Pinto-Alphandary H, Gouritin B, Lacur B, Farinotti R, et al. Insulin-loaded nanocapsules for oral administration: in vitro and in vivo investigation. Drug Dev Res. 2000;49:109–17.
Sadeghi AM, Dorkoosh FA, Avadi MR, Weinhold M, Bayat A, Delie F, et al. Permeation enhancer effect of chitosan and chitosan derivatives: comparison of formulations as soluble polymers and nanoparticulate systems on insulin absorption in Caco-2 cells. Eur J Pharm Biopharm. 2008;70:270–8.
Ponchel G, Montisci M-J, Dembri A, Durrer C, Duchene D. Mucoadhesion of colloidal particulate systems in the gastrointestinal tract. Eur J Pharm Biopharm. 1997;44:25–31.
Hans ML, Lowman AM. Biodegradable nanoparticles for drug delivery and targeting. Curr Opin Solid State Mater Sci. 2002;6:319–27.
Janes KA, Calvo P, Alonso MJ. Polysaccharide colloidal particles as delivery systems for macromolecules. Adv Drug Del Rev. 2001;47:83–57.
Goycoolea FM, Lollo G, Remuñán-López C, Quaglia F, Alonso MJ. Chitosan-alginate blended nanoparticles as carriers for the transmucosal delivery of macromolecules. Biomacromolecules. 2009;10:1736–43.
Kotzé AF, de Leeuw BJ, Lueßen HL, de Boer AG, Verhoef JC, Junginger HE. Chitosans for enhanced delivery of therapeutic peptides across intestinal epithelia: in vitro evaluation in Caco-2 cell monolayers. Int J Pharm. 1997;159:131–6.
Thanou M, Verhoef JC, Roemeijn SG, Nagelkerke JF, Merkus WH, Junginger HE. Effects of N-trimethyl chitosan chloride, a novel absorption enhancer, on Caco-2 intestinal epithelia and the ciliary beat frequency of chicken embryo trachea. Int J Pharm. 1999;185:73–8.
Sandri G, Bonferoni MC, Rossi S, Ferrari F, Gibin S, Zambito Y, et al. Nanoparticles based on N-trimethyl chitosan: evaluation of absorption properties using in vitro (Caco-2 cells) and ex vivo (excised rat jejunum) models. Eur J Pharm Biopharm. 2007;65:78–67.
Sieval AB, Thanou M, Kotzè AF, Verhoef JC, Brussee J, Junginger HE. Preparation and NMR characterization of highly substituted N-trimethyl chitosan chloride. Carbohyd Res. 1998;36:175–65.
Di Colo G, Burgalassi S, Zambito Y, Monti D, Chetoni P. Effects of different N-trimethyl chitosans on in vitro/in vivo ofloxacin transcorneal permeation. J Pharm Sci. 2004;93:2851–62.
Calvo P, Remunan-Lopez C, Vila-Jato JL, Alonso MJ. Novel hydrophilic chitosan–polyethylene oxide nanoparticles as protein carriers. J Appl Polym Sci. 1997;63:125–32.
Dorkoosh FA, Verhoef JC, Ambagts MHC, Rafiee-Tehrani M, Borchard G, Junginger HE. Peroral delivery systems based on superporous hydrogel polymers: release characteristics for the peptide drugs buserelin, octreotide and insulin. Eur J Pharm Sci. 2002;15:433–9.
Dodane V, Amin Khan A, Mervin JR. Effect of chitosan on epithelial permeability and structure. Int J Pharm. 1999;182:21–32.
Smith J, Wood E, Dornish M. Effect of chitosan on epithelial cell tight junctions. Pharm Res. 2004;21:43–9.
Ma Z, Lim L-Y. Uptake of chitosan associated insulin in Caco-2 cell monolayers: a comparison between chitosan molecules and chitosan nanoparticles. Pharm Res. 2003;20:1812–9.
Bayat A, Dorkoosh FA, Dehpour AR, Moezi L, Larijani B, Junginger HE, et al. Nanoparticles of quaternized chitosan derivatives as a carrier for colon delivery of insulin: ex vivo and in vitro study. Int J Pharm. 2008;356:259–66.
Yin L, Ding J, He C, Cui L, Tang C, Yin C. Drug permeability and mucoadhesion properties of thiolated trimethyl chitosan nanoparticles in oral insulin delivery. Biomaterials. 2009;30:5691–700.
Jonker C, Hamman JH, Kotze AF. Intestinal paracellular permeation enhancement with quaternized chitosan: in situ and in vitro evaluation. Int J Pharm. 2002;238:205–13.
Lee K-J, Johnson N, Castelo J, Sinko PJ, Grass G, Holme K, et al. Effect of experimental pH on the in vitro permeability in intact rabbit intestines and Caco-2 monolayer. Eur J Pharm Sci. 2005;25:193–200.
Horter D, Dressman JB. Influence of physicochemical properties on dissolution of drugs in the gastrointestinal tract. Adv Drug Deliv Rev. 1997;25:3–14.
Schillin RJ, Mitra AK. Intestinal mucosal transport of insulin. Int J Pharm. 1990;62:53–64.
Aoki Y, Morishita M, Asai K, Akikusa B, Hosoda S, Takayama K. Region-dependent role of the mucous/glycocalyx layers in insulin permeation across rat small intestinal membrane. Pharm Res. 2005;22:1854–62.
Aoki Y, Morishita M, Takayama K. Role of the mucous/glycocalyx layers in insulin permeation across the rat ileal membrane. Int J Pharm. 2005;297:98–109.
Morishita M, Aoki Y, Sakagami M, Nagai T, Takayama K. In situ ileal absorption of insulin in rats: effects of hyaluronidase pretreatment diminishing the mucous/glycocalyx layers. Pharm Res. 2004;21:309–16.
Sarmento B, Ribeiro A, Veiga F, Ferreira D, Neufeld R. Oral bioavailability of insulin contained in polysaccharide nanoparticles. Biomacromolecules. 2007;8:3054–60.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sandri, G., Bonferoni, M.C., Rossi, S. et al. Insulin-Loaded Nanoparticles Based on N-Trimethyl Chitosan: In Vitro (Caco-2 Model) and Ex Vivo (Excised Rat Jejunum, Duodenum, and Ileum) Evaluation of Penetration Enhancement Properties. AAPS PharmSciTech 11, 362–371 (2010). https://doi.org/10.1208/s12249-010-9390-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-010-9390-3