Abstract
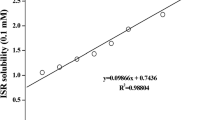
The main aim of the present study was to evaluate potential of ternary complexation (comprising of drug, cyclodextrin and polymer) as an approach for taste masking. For this purpose famotidine with property of bitter taste was selected as a model drug. Improvement in taste masking capability of cyclodextrin towards famotidine was evaluated by formulating a ternary complex including hydrophilic polymer hydroxyl propyl methyl cellulose (HPMC 5 cps) as the third component. Phase solubility analysis at 25 °C was carried out for both the binary systems (viz. drug–cyclodextrin and drug–polymer) and the ternary system (drug–cyclodextrin–polymer). Ternary complex was prepared using solution method and was further characterized using XRD, DSC, FT-IR and microscopic studies. In vitro dissolution study was carried out to see the effect of ternary complexation on drug release. Taste perception study was carried out on human volunteers to evaluate the taste masking ability of ternary complexation. Results obtained from phase solubility analysis showed that the combined use of polymer and cyclodextrin effectively increased the stability constant of the complex [from 538 M−1 for binary system to 15,096 M−1 for ternary system]. Ternary system showed effective taste masking as compared to binary complex and at the same time showed no limiting effect on the drug release (D.E15min = 90%). The effective taste masking was attributed to the enhanced complexation of famotidine in ternary system compared to binary system and the same was confirmed from the characterization studies. In conclusion, the study confirmed that ternary complexation can be utilized as an alternative approach for effective taste masking.







Similar content being viewed by others
References
T. Loftsson, and M. E. Brewster. Pharmaceutical applications of cyclodextrins: 1. Drug solubilization and stabilization. J. Pharm. Sci. 85:1017–1025 (1996).
H. Sohi, Y. Sultana, and R. Khar. Taste masking technologies in oral pharmaceuticals: Recent developments and approaches. Drug Dev. Ind. Pharm. 30:429–448 (2004).
J. Szejtli. Past, present, and future of cyclodextrin research. Pure Appl. Chem. 76:1825–1845 (2004).
C. Giancarlo, B. Arianna, B. Enzo, C. Paolo, and F. Trotta. Cyclodextrins as food additives and in food processing. Curr. Nutri. Food Sci. 2:343–350 (2006).
F. Noriaki, U. Ikumi, O. Takashi, H. Shun, and N. Saburo. Masking mechanisms of bitter taste of drugs studied with ion selective electrodes. Chem. Pharm. Bull. 54:1155–1161 (2006).
R. A. Rajewski, and V. J. Stella. Pharmaceutical applications of cyclodextrins II: In vivo drug delivery. J. Pharm. Sci. 85:1142–1169 (1996).
K. Okimoto, R. A. Rajewski, J. A. Jona, and V. J. Stella. The interaction of charged and uncharged drugs with a neutral (HP-b-CD) and anionically charged (SBE7-b-CD) b-cyclodextrin. Pharm. Res. 13:256–264 (1996).
V. J. Stella, and R. A. Rajewski. Cyclodextrins: Their future in drug formulation and delivery. Pharm. Res. 14:556–567 (1997).
V. J. Stella, V. M. Rao, E. A. Zannou, and V. Zia. Mechanisms of drug release from cyclodextrin complexes. Adv. Drug Del. Rev. 36:3–16 (1999).
A. R. Patel, and P. R. Vavia. Effect of hydrophilic polymers on solubilization of fenofibrate by cyclodextrin complexation. J. Incl. Phenom. Macrocycl. Chem. 56:247–251 (2006).
T. Loftsson, and H. Frioriksdottir. The effect of water-soluble polymers on the aqueous solubility and complexing abilities of b-cyclodextrin. Int. J. Pharm. 163:115–121 (1998).
A. M. Sigurdardottir, and T. Loftsson. The effect of polyvinylpyrrolidone on cyclodextrin complexation of hydrocortisone and its diffusion through hairless mouse skin. Int. J. Pharm. 126:73–78 (1995).
R. C. Rowe, J. S. Paul, and J. W. Paul. Handbook of Pharmaceutical Excipients, Pharmaceutical Press, London, 2003.
S. P. Li, S. A. Martellucci, R. D. Bruce, A. C. Kinyon, M. B. Hay, and J. D. Higgins. Evaluation of the film-coating properties of a hydroxyethyl cellulose/hydroxypropyl methylcellulose polymer system. Drug Dev. Ind. Pharm. 28:389–401 (2002).
T. Higuchi, and K. A. Connors. Phase solubility techniques. Adv. Anal. Chem. Instrum. 4:117–118 (1965).
K. A. Khan. The concept of dissolution efficiency. J. Pharm. Pharmacol. 27:48–49 (1975).
F. Acarturk, O. Kislal, and N. Celebi. The effect of some natural polymers on the solubility and dissolution characteristics of nifedipine. Int. J. Pharm. 85:1–15 (1992).
T. Loftsson, H. Frikdriksdottir, A. M. Sigurkdardottir, and H. Ueda. Evaluation of cyclodextrin solubilization of drugs. Int. J. Pharm. 110:169–177 (1994).
M. Y. Rekharshy, and Y. Inoue. Detection of paramagnetic pH dependent Inclusion complexes. Chem. Rev. 98:1875–1896 (1998).
G. S. Jadhav, A. R. Patel, P. R. Vavia, A. K. Malde, and E. C. Coutinho. Interaction of valdecoxib with β-cyclodextrin: Experimental and molecular modeling studies. J. Incl. Phenom. Macrocycl. Chem. 56:247–251 (2006).
V. R. Sinha, R. Anitha, S. Ghosh, A. Nanda, and R. Kumria. Complexation of celecoxib with β-cyclodextrin: Characterization of the interaction in solution and in solid state. J. Pharm. Sci. 94:676–687 (2005).
B. George, and P. Mcintyre. Introduction to spectrum interpretation. In D. J. Mowthorpe (ed.), Infrared Spectroscopy: Analytical Chemistry by Open Learning, Wiley, London, UK, 1987, pp. 161–201.
Acknowledgement
Authors wish to thank University Grant Commission (UGC), India for providing financial assistance through senior research fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patel, A.R., Vavia, P.R. Preparation and Evaluation of Taste Masked Famotidine Formulation Using Drug/β-cyclodextrin/Polymer Ternary Complexation Approach. AAPS PharmSciTech 9, 544–550 (2008). https://doi.org/10.1208/s12249-008-9078-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-008-9078-0