Abstract
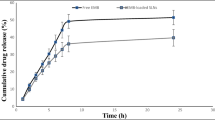
This investigation was undertaken to evaluate practical feasibility of site specific pulmonary delivery of liposomal encapsulated Dapsone (DS) dry powder inhaler for prolonged drug retention in lungs as an effective alternative in prevention of Pneumocystis carinii pneumonia (PCP) associated with immunocompromised patients. DS encapsulated liposomes were prepared by thin film evaporation technique and resultant liposomal dispersion was passed through high pressure homogenizer. DS nano-liposomes (NLs) were separated by ultra centrifugation and characterized. NLs were dispersed in phosphate buffer saline (PBS) pH 7.4 containing different carriers like lactose, sucrose, and hydrolyzed gelatin, and 15% l-leucine as antiadherent. The resultant dispersion was spray dried and spray dried formulation were characterized to ascertain its performance. In vitro pulmonary deposition was assessed using Andersen Cascade Impactor as per USP. NLs were found to have average size of 137 ± 15 nm, 95.17 ± 3.43% drug entrapment, and zeta potential of 0.8314 ± 0.0827 mV. Hydrolyzed gelatin based formulation was found to have low density, good flowability, particle size of 7.9 ± 1.1 μm, maximum fine particle fraction (FPF) of 75.6 ± 1.6%, mean mass aerodynamic diameter (MMAD) 2.2 ± 0.1 μm, and geometric standard deviation (GSD) 2.3 ± 0.1. Developed formulations were found to have in vitro prolonged drug release up to 16 h, and obeys Higuchi's Controlled Release model. The investigation provides a practical approach for direct delivery of DS encapsulated in NLs for site specific controlled and prolonged release behavior at the site of action and hence, may play a promising role in prevention of PCP.




Similar content being viewed by others
REFERENCES
E. A. Ashley, M. A. Johnson, and M. C. I. Lipman. Human immunodeficiency virus and respiratory infection. Curr. Opin. Pulm. Med. 6:240–245 (2000).
J. F. Murray, and J. Mills. Pulmonary infectious complications of human immunodeficiency virus infection. Part I. Am. Rev. Respir. Dis. 141(5 Pt 1):1356–1372 (1990).
J. A. Fishman. Treatment of infection due to Pneumocystis carinii. Antimicrob. Agents Chemother. 426:1309–1314 (1998).
J. E. Gallant, R. E. Chaisson, and R. D. Moore. The effect of adjunctive corticosteroids for the treatment of Pneumocystis carinii pneumonia on mortality and subsequent complications. Chest 114(5):1230–1231 (1998) Nov.
B. S. Mathew, and S. A. Grossman. Pneumocystis carinii pneumonia prophylaxis in HIV negative patients with primary CNS lymphoma. Cancer Treat. Rev. 29:105–119 (2003).
J. C. Waldrep. New aerosol drug delivery systems for the treatment of immune-mediated pulmonary diseases. Drugs Today 3(6):549–561 (1998).
S. Ashurst, A. Malton, D. Prime, et al. Latest advances in the development of dry powder inhaler. Pharm. Sci. Tech. Today 3(7):246–256 (2000).
G. V. Letsou, H. J. Safi, M. J. Reardon, et al. Pharmacokinetics of liposomal aerosolized cyclosporine A for pulmonary immunosuppression. Ann. Thorac. Surg 68:2044–2048 (1999).
M. B. Chougule, B. Padhi, and A. N. Misra. Nanoliposomal dry powder inhaler formulation of Amiloride hydrochloride. J. Nanosci. Nanotechnol. 6:3001–3009 (2006).
Y. Lo, J. Tsai, and J. Kuo. Liposomes and disaccharides as carriers in spray-dried powder formulations of superoxide dismutase. J. Control. Release 94:259–272 (2004).
D. Lu, and A. J. Hickey. Liposomal dry powders as aerosols for pulmonary delivery of proteins. AAPS PharmSciTech. 64:E641–E648 (2005).
C. Vermehren, S. Frokjaer, T. Aurstad, et al. Lung surfactant as a drug delivery system. Int. J. Pharm. 307(1):89–92 (2006).
S. P. Shah, and A. N. Misra. Development of liposomal Amphotericin B dry powder inhaler formulation. Drug Deliv. 11(4):247–253 (2004).
M. Joshi, and A. N. Misra. Disposition kinetics of ketotifen from liposomal dry powder for inhalation in rat lung. Clin. Exp. Pharmacol. Physiol. 30(3):153–156 (2003).
M. B. Chougule, B. Padhi, and A. N. Misra. Nano-liposomal dry powder inhaler of tacrolimus: preparation, characterization and pulmonary pharmacokinetics. Int. J. Nanomed. 2(4):1–17 (2007).
M. R. Joshi, and A. Misra. Liposomal budesonide for dry powder inhaler: preparation and stabilization. AAPS PharmSciTech. 2(4):25 (2001).
R. R. C. New. Preparation of liposomes. In R. R. C. New (ed.), Liposomes a practical approach, Oxford University Press, New York, 1990.
L. Mu, and S. S. Feng. Fabrication, characterization and in vitro release of paclitaxel (Taxol) loaded poly (lactic-co-glycolic acid) microspheres prepared by spray drying technique with lipid/cholesterol emulsifiers. J. Control Release 76:239–254 (2001).
J. Mojaverian, W. A. Rosen, S. Vadino, et al. In-vivo/in-vitro correlation of four extended release formulations of pseudoephedrine sulfate. J. Pharm. Biomed. Anal. 15:439–445 (1997).
V. S. Kharasch, T. D. Sweeney, J. Fredberg, et al. Pulmonary surfactant as a vehicle for intratracheal delivery of technetium sulfur colloid and pentamidine in hamster lungs. Am. Rev. Respir. Dis. 144(4):909–913 (1991).
W. J. Davidson, D. Dorscheid, R. Spragg. Exogenous pulmonary surfactant for the treatment of adult patients with acute respiratory distress syndrome: results of a meta-analysis. Crit. Care. 10:R41 (2006).
J. Elversson, A. Millqvist-Fureby, G. Alderborn, et al. Droplet and particle size relationship and shell thickness of inhalable lactose particles during spray drying. J. Pharm. Sci. 92(4):900–910 (2003).
X. C. Nguyen, J. D. Herberger, and P. A. Burke1. Protein powders for encapsulation: a comparison of spray-freeze drying and spray drying of darbepoetin alfa. Pharm. Res. 21(3):507–514 (2004).
C. Bosquillon, P. G. Rouxhet, F. Ahimou, et al. Aerosolization properties, surface composition and physical state of spray-dried protein powders. J. Control Release 99(3):357–367 (2004).
R. Vanbever, J. Mintzes, J. Wan, et al. Formulation and physical characterization of large porous particles for inhalation. Pharm. Res. 16:1735–1742 (1999).
N. Tsapis, D. Bennet, B. Jackson, et al. Trojan particles: large porous carriers of nanoparticles for drug delivery. Proc. Nat. Acad. Sci. U. S. A. 99:12001–12005 (2002).
H. Steckel, and H. G. Brandes. A novel spray-drying technique to produce low density particles for pulmonary delivery. Int. J. Pharm. 278:187–195 (2004).
Y. Lo, J. Tsai, and J. Kuo. Liposomes and disaccharides as carriers in spray-dried powder formulations of superoxide dismutase. J. Control Release 94:259–272 (2004).
N. Y. K. Chew, P. Tang, H. K. Chan, et al. How much particle surface corrugation is sufficient to improve aerosol performance of powders? Pharm. Res. 22:148–152 (2005).
ACKNOWLEDGMENTS
The authors are thankful to Indian Council of Medical Research (ICMR), New Delhi, India for providing funding to the research project and Technology Information and Forecasting Council's (TIFAC) Centre of Relevance and Excellence in New Drug Delivery System. The authors are thankful to Sun Pharmaceutical Advanced Research Center (SPARC), Vadodara, India, Zydus Cadila Ltd, Ahmedabad, India and Panacea Biotec Ltd, Lalru, India for availing facilities for part of research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chougule, M., Padhi, B. & Misra, A. Development of Spray Dried Liposomal Dry Powder Inhaler of Dapsone. AAPS PharmSciTech 9, 47–53 (2008). https://doi.org/10.1208/s12249-007-9024-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-007-9024-6