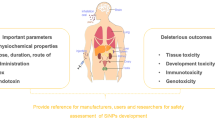
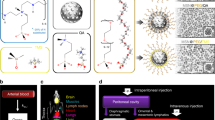
Abstract
Over the past few years, nanoparticles have drawn particular attention in designing and developing drug delivery systems due to their distinctive advantages like improved pharmacokinetics, reduced toxicity, and specificity. Along with other successful nanosystems, silica nanoparticles (SNPs) have shown promising effects for therapeutic and diagnostic purposes. These nanoparticles are of great significance owing to their modifiable surface with various ligands, tunable particle size, and large surface area. The rate and extent of degradation and clearance of SNPs depend on factors such as size, shape, porosity, and surface modification, which directly lead to varying toxic mechanisms. Despite SNPs’ enormous potential for clinical and pharmaceutical applications, safety concerns have hindered their translation into the clinic. This review discusses the biodistribution, toxicity, and clearance of SNPs and the formulation-related factors that ultimately influence clinical efficacy and safety for treatment. A holistic view of SNP safety will be beneficial for developing an enabling SNP-based drug product.
Graphical Abstract



Similar content being viewed by others
Data Availability
This is a review article without original data collected. All the cited data were documented with the original source.
Abbreviations
- EPR:
-
Enhanced permeability and retention
- FITC:
-
Fluorescein isothiocyanate
- HUVEC:
-
Human umbilical vein endothelial cells
- ID:
-
Injected dose
- IL:
-
Interleukin
- LDH:
-
Lactate dehydrogenase
- MAPK:
-
Mitogen-activated protein kinase
- MSN:
-
Mesoporous silica nanoparticles
- MTD:
-
Maximum tolerated dose
- NF-κB:
-
Nuclear factor kappa B
- NOX2:
-
NADPH oxidase 2
- PEG:
-
Polyethylene glycol
- RES:
-
Reticuloendothelial systems
- RNS:
-
Reactive nitrogen species
- ROS:
-
Reactive oxygen species
- SNP:
-
Silica nanoparticle
- TEM:
-
Transmission electron microscopy
- TNF:
-
Tumor necrosis factor
- TXNIP:
-
Thioredoxin-interacting protein
References
Ren X, Cheng S, Liang Y, Yu X, Sheng J, Wan Y, et al. Mesoporous silica nanospheres as nanocarriers for poorly soluble drug itraconazole with high loading capacity and enhanced bioavailability. Microporous Mesoporous Mater. 2020;305:110389.
Yusuf A, Almotairy ARZ, Henidi H, Alshehri OY, Aldughaim MS. Nanoparticles as drug delivery systems: a review of the implication of nanoparticles’ physicochemical properties on responses in biological systems. Polymers. 2023;15(7):1596.
Yuan H, Guo H, Luan X, He M, Li F, Burnett J, et al. Albumin nanoparticle of paclitaxel (abraxane) decreases while taxol increases breast cancer stem cells in treatment of triple negative breast cancer. Mol Pharm. 2020;17(7):2275–86.
Zhong H, Chan G, Hu Y, Hu H, Ouyang D. A comprehensive map of FDA-approved pharmaceutical products. Pharmaceutics. 2018;10(4):263.
Zhou Y, Quan G, Wu Q, Zhang X, Niu B, Wu B, et al. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharmaceutica Sinica B. 2018;8(2):165–77.
Xu B, Li S, Shi R, Liu H. Multifunctional mesoporous silica nanoparticles for biomedical applications. Signal Transduct Target Ther. 2023;8(1):435.
Watermann A, Brieger J. Mesoporous silica nanoparticles as drug delivery vehicles in cancer. Nanomaterials. 2017;7(7):189.
Janßen HC, Angrisani N, Kalies S, Hansmann F, Kietzmann M, Warwas DP, et al. Biodistribution, biocompatibility and targeted accumulation of magnetic nanoporous silica nanoparticles as drug carrier in orthopedics. J Nanobiotechnol. 2020;18(1):14.
Chen W-H, Luo G-F, Lei Q, Cao F-Y, Fan J-X, Qiu W-X, et al. Rational design of multifunctional magnetic mesoporous silica nanoparticle for tumor-targeted magnetic resonance imaging and precise therapy. Biomaterials. 2016;76:87–101.
Lee SB, Lee HW, Darmawan BA, Lee I-K, Cho SJ, Chin J, et al. NIR dye-loaded mesoporous silica nanoparticles for a multifunctional theranostic platform: visualization of tumor and ischemic lesions, and performance of photothermal therapy. J Ind Eng Chem. 2020;88:99–105.
Phillips E, Penate-Medina O, Zanzonico PB, Carvajal RD, Mohan P, Ye Y, et al. Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe. Sci Transl Med. 2014;6(260):260ra149-260ra149.
Select Committee on GRAS Substances (SCOGS) Opinion: Silicates. 2015. http://wayback.archive-it.org/7993/20171031063508/https://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/SCOGS/ucm260849.htm.
Janjua TI, Cao Y, Yu C, Popat A. Clinical translation of silica nanoparticles. Nat Rev Mater. 2021;6(12):1072–4.
Murugadoss S, Lison D, Godderis L, Van Den Brule S, Mast J, Brassinne F, et al. Toxicology of silica nanoparticles: an update. Arch Toxicol. 2017;91(9):2967–3010.
Chen L, Liu J, Zhang Y, Zhang G, Kang Y, Chen A, et al. The toxicity of silica nanoparticles to the immune system. Nanomedicine. 2018;13(15):1939–62.
Wei Y, Quan L, Zhou C, Zhan Q. Factors relating to the biodistribution & clearance of nanoparticles & their effects on in vivo application. Nanomedicine. 2018;13(12):1495–512.
Jasinski DL, Li H, Guo P. The effect of size and shape of RNA nanoparticles on biodistribution. Mol Ther. 2018;26(3):784–92.
Bagwe RP, Hilliard LR, Tan W. Surface modification of silica nanoparticles to reduce aggregation and nonspecific binding. Langmuir. 2006;22(9):4357–62.
Dong X, Wu Z, Li X, Xiao L, Yang M, Li Y, et al. The size-dependent cytotoxicity of amorphous silica nanoparticles: a systematic review of in vitro studies. Int J Nanomedicine. 2020;15:9089–113.
Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, et al. Analysis of nanoparticle delivery to tumours. Nat Rev Mater. 2016;1(5):16014.
Mirkasymov AB, Zelepukin IV, Nikitin PI, Nikitin MP, Deyev SM. In vivo blockade of mononuclear phagocyte system with solid nanoparticles: efficiency and affecting factors. J Control Release. 2021;330:111–8.
Licciardello N, Hunoldt S, Bergmann R, Singh G, Mamat C, Faramus A, et al. Biodistribution studies of ultrasmall silicon nanoparticles and carbon dots in experimental rats and tumor mice. Nanoscale. 2018;10(21):9880–91.
Ferreira CA, Goel S, Ehlerding EB, Rosenkrans ZT, Jiang D, Sun T, et al. Ultrasmall porous silica nanoparticles with enhanced pharmacokinetics for cancer theranostics. Nano Lett. 2021;21(11):4692–9.
Xie G, Sun J, Zhong G, Shi L, Zhang D. Biodistribution and toxicity of intravenously administered silica nanoparticles in mice. Arch Toxicol. 2010;84(3):183–90.
Tassinari R, Martinelli A, Valeri M, Maranghi F. Amorphous silica nanoparticles induced spleen and liver toxicity after acute intravenous exposure in male and female rats. Toxicol Ind Health. 2021;37(6):328–35.
Bhavsar D, Patel V, Sawant K. Systematic investigation of in vitro and in vivo safety, toxicity and degradation of mesoporous silica nanoparticles synthesized using commercial sodium silicate. Microporous Mesoporous Mater. 2019;284:343–52.
Liu T, Li L, Teng X, Huang X, Liu H, Chen D, et al. Single and repeated dose toxicity of mesoporous hollow silica nanoparticles in intravenously exposed mice. Biomaterials. 2011;32(6):1657–68.
Kim I-Y, Joachim E, Choi H, Kim K. Toxicity of silica nanoparticles depends on size, dose, and cell type. Nanomed: Nanotechnol Biol Med. 2015;11(6):1407–16.
Zhao Y, Wang Y, Ran F, Cui Y, Liu C, Zhao Q, et al. A comparison between sphere and rod nanoparticles regarding their in vivo biological behavior and pharmacokinetics. Sci Rep. 2017;7(1):4131.
MadathiparambilVisalakshan R, González García LE, Benzigar MR, Ghazaryan A, Simon J, Mierczynska-Vasilev A, et al. The influence of nanoparticle shape on protein corona formation. Small. 2020;16(25):2000285.
Wang W, Gaus K, Tilley RD, Gooding JJ. The impact of nanoparticle shape on cellular internalisation and transport: what do the different analysis methods tell us? Mater Horiz. 2019;6(8):1538–47.
Wani A, Savithra GHL, Abyad A, Kanvinde S, Li J, Brock S, et al. Surface PEGylation of mesoporous silica nanorods (MSNR): effect on loading, release, and delivery of mitoxantrone in hypoxic cancer cells. Sci Rep. 2017;7(1):2274.
Sargazi S, Laraib U, Barani M, Rahdar A, Fatima I, Bilal M, et al. Recent trends in mesoporous silica nanoparticles of rode-like morphology for cancer theranostics: a review. J Mol Struct. 2022;1261:132922.
Shao D, Lu M-m, Zhao Y-w, Zhang F, Tan Y-f, Zheng X, et al. The shape effect of magnetic mesoporous silica nanoparticles on endocytosis, biocompatibility and biodistribution. Acta Biomaterialia. 2017;49:531–40.
Fröhlich E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int J Nanomedicine. 2012;7:5577–91.
Dogra P, Adolphi NL, Wang Z, Lin Y-S, Butler KS, Durfee PN, et al. Establishing the effects of mesoporous silica nanoparticle properties on in vivo disposition using imaging-based pharmacokinetics. Nat Commun. 2018;9(1):4551.
Lei Q, Guo J, Noureddine A, Wang A, Wuttke S, Brinker CJ, et al. Sol–Gel-based advanced porous silica materials for biomedical applications. Adv Func Mater. 2020;30(41):1909539.
Bakhshian Nik A, Zare H, Razavi S, Mohammadi H, Torab Ahmadi P, Yazdani N, et al. Smart drug delivery: capping strategies for mesoporous silica nanoparticles. Microporous Mesoporous Mater. 2020;299:110115.
Mohammadpour R, Cheney DL, Grunberger JW, Yazdimamaghani M, Jedrzkiewicz J, Isaacson KJ, et al. One-year chronic toxicity evaluation of single dose intravenously administered silica nanoparticles in mice and their Ex vivo human hemocompatibility. J Control Release. 2020;324:471–81.
Yu T, Greish K, McGill LD, Ray A, Ghandehari H. Influence of geometry, porosity, and surface characteristics of silica nanoparticles on acute toxicity: their vasculature effect and tolerance threshold. ACS Nano. 2012;6(3):2289–301.
Bindini E, Ramirez MdlA, Rios X, Cossío U, Simó C, Gomez-Vallejo V, et al. In vivo tracking of the degradation of mesoporous silica through 89Zr radio-labeled core–shell nanoparticles. Small. 2021;17(30):2101519.
Tishchenko VK, Petriev VM, Mikhailovskaya AA, Smoryzanova OA, Kabashin AV, Zavestovskaya IN. Ex vivo biodistribution of gallium-68-labeled porous silicon nanoparticles. J Phys: Conf Ser. 2020;1439(1):012035.
Peng H, Xu Z, Wang Y, Feng N, Yang W, Tang J. Biomimetic mesoporous silica nanoparticles for enhanced blood circulation and cancer therapy. ACS Appl Bio Mater. 2020;3(11):7849–57.
Yang B, Zhou S, Zeng J, Zhang L, Zhang R, Liang K, et al. Super-assembled core-shell mesoporous silica-metal-phenolic network nanoparticles for combinatorial photothermal therapy and chemotherapy. Nano Res. 2020;13(4):1013–9.
Castillo RR, Vallet-Regí M. Functional mesoporous silica nanocomposites: biomedical applications and biosafety. Int J Mol Sci. 2019;20(4):929.
Hu Y, Niemeyer CM. Designer DNA–silica/carbon nanotube nanocomposites for traceable and targeted drug delivery. J Mater Chem B. 2020;8(11):2250–5.
Park JH, Jackman JA, Ferhan AR, Belling JN, Mokrzecka N, Weiss PS, et al. Cloaking silica nanoparticles with functional protein coatings for reduced complement activation and cellular uptake. ACS Nano. 2020;14(9):11950–61.
Suk JS, Xu Q, Kim N, Hanes J, Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99(Pt A):28–51.
Abdollah MRA, Carter TJ, Jones C, Kalber TL, Rajkumar V, Tolner B, et al. Fucoidan prolongs the circulation time of dextran-coated iron oxide nanoparticles. ACS Nano. 2018;12(2):1156–69.
Sindhwani S, Syed AM, Ngai J, Kingston BR, Maiorino L, Rothschild J, et al. The entry of nanoparticles into solid tumours. Nat Mater. 2020;19(5):566–75.
Adumeau L, Genevois C, Roudier L, Schatz C, Couillaud F, Mornet S. Impact of surface grafting density of PEG macromolecules on dually fluorescent silica nanoparticles used for the in vivo imaging of subcutaneous tumors. Biochim Biophys Acta (BBA) - Gen Subj. 2017;1861(6):1587–96.
Hayashi Y, Takamiya M, Jensen PB, Ojea-Jiménez I, Claude H, Antony C, et al. Differential nanoparticle sequestration by macrophages and scavenger endothelial cells visualized in vivo in real-time and at ultrastructural resolution. ACS Nano. 2020;14(2):1665–81.
Kramer L, Winter G, Baur B, Kuntz AJ, Kull T, Solbach C, et al. Quantitative and correlative biodistribution analysis of 89Zr-labeled mesoporous silica nanoparticles intravenously injected into tumor-bearing mice. Nanoscale. 2017;9(27):9743–53.
Sudimack J, Lee RJ. Targeted drug delivery via the folate receptor. Adv Drug Deliv Rev. 2000;41(2):147–62.
Zwicke GL, Ali Mansoori G, Jeffery CJ. Utilizing the folate receptor for active targeting of cancer nanotherapeutics. Nano Rev. 2012;3(1):18496.
Zhang Y, Ye Z, He R, Li Y, Xiong B, Yi M, et al. Bovine serum albumin-based and dual-responsive targeted hollow mesoporous silica nanoparticles for breast cancer therapy. Colloids Surf B. 2023;224:113201.
Xu X, Wu C, Bai A, Liu X, Lv H, Liu Y. Folate-functionalized mesoporous silica nanoparticles as a liver tumor-targeted drug delivery system to improve the antitumor effect of paclitaxel. J Nanomater. 2017;2017:2069685.
Song Y, Zhou B, Du X, Wang Y, Zhang J, Ai Y, et al. Folic acid (FA)-conjugated mesoporous silica nanoparticles combined with MRP-1 siRNA improves the suppressive effects of myricetin on non-small cell lung cancer (NSCLC). Biomed Pharmacother. 2020;125:109561.
Li Z, Zhang Y, Zhu C, Guo T, Xia Q, Hou X, et al. Folic acid modified lipid-bilayer coated mesoporous silica nanoparticles co-loading paclitaxel and tanshinone IIA for the treatment of acute promyelocytic leukemia. Int J Pharm. 2020;586:119576.
Pochert A, Vernikouskaya I, Pascher F, Rasche V, Lindén M. Cargo-influences on the biodistribution of hollow mesoporous silica nanoparticles as studied by quantitative 19F-magnetic resonance imaging. J Colloid Interface Sci. 2017;488:1–9.
Wang Y, Cheng W, Zhu J, He L, Ren W, Bao D, et al. Programmed Co-delivery of tamoxifen and docetaxel using lipid-coated mesoporous silica nanoparticles for overcoming CYP3A4-mediated resistance in triple-negative breast cancer treatment. Biomed Pharmacother. 2024;170:116084.
Yin H, Yan Q, Liu Y, Yang L, Liu Y, Luo Y, et al. Co-encapsulation of paclitaxel and 5-fluorouracil in folic acid-modified, lipid-encapsulated hollow mesoporous silica nanoparticles for synergistic breast cancer treatment. RSC Adv. 2022;12(50):32534–51.
Pei W, Cai L, Gong X, Zhang L, Zhang J, Zhu P, et al. Drug-loaded oleic-acid grafted mesoporous silica nanoparticles conjugated with α-lactalbumin resembling BAMLET-like anticancer agent with improved biocompatibility and therapeutic efficacy. Mater Today Bio. 2022;15:100272.
Noureddine A, Marwedel B, Tang L, Medina LY, Serda RE. Specific tumor localization of immunogenic lipid-coated mesoporous silica nanoparticles following intraperitoneal administration in a mouse model of serous epithelial ovarian cancer. Cancers. 2023;15(18):4626.
Maeda H, Nakamura H, Fang J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv Drug Deliv: Perspect Prospects. 2013;65(1):71–9.
Kingston BR, Lin ZP, Ouyang B, MacMillan P, Ngai J, Syed AM, et al. Specific endothelial cells govern nanoparticle entry into solid tumors. ACS Nano. 2021;15(9):14080–94.
Maggini L, Cabrera I, Ruiz-Carretero A, Prasetyanto EA, Robinet E, De Cola L. Breakable mesoporous silica nanoparticles for targeted drug delivery. Nanoscale. 2016;8(13):7240–7.
Bansal A, Simon MC. Glutathione metabolism in cancer progression and treatment resistance. J Cell Biol. 2018;217(7):2291–8.
Kennedy L, Sandhu JK, Harper M-E, Cuperlovic-Culf M. Role of glutathione in cancer: from mechanisms to therapies. Biomolecules. 2020;10(10):1429.
Ding X, Yu W, Wan Y, Yang M, Hua C, Peng N, et al. A pH/ROS-responsive, tumor-targeted drug delivery system based on carboxymethyl chitin gated hollow mesoporous silica nanoparticles for anti-tumor chemotherapy. Carbohyd Polym. 2020;245:116493.
Benfeitas R, Bidkhori G, Mukhopadhyay B, Klevstig M, Arif M, Zhang C, et al. Characterization of heterogeneous redox responses in hepatocellular carcinoma patients using network analysis. eBioMedicine. 2019;40:471–87.
He Y, Shao L, Hu Y, Zhao F, Tan S, He D, et al. Redox and pH dual-responsive biodegradable mesoporous silica nanoparticle as a potential drug carrier for synergistic cancer therapy. Ceram Int. 2021;47(4):4572–8.
Liu J, Luo Z, Zhang J, Luo T, Zhou J, Zhao X, et al. Hollow mesoporous silica nanoparticles facilitated drug delivery via cascade pH stimuli in tumor microenvironment for tumor therapy. Biomaterials. 2016;83:51–65.
Du L, Liao S, Khatib HA, Stoddart JF, Zink JI. Controlled-access hollow mechanized silica nanocontainers. J Am Chem Soc. 2009;131(42):15136–42.
Qi G, Shi G, Wang S, Hu H, Zhang Z, Yin Q, et al. A novel pH-responsive iron oxide core-shell magnetic mesoporous silica nanoparticle (M-MSN) system encapsulating doxorubicin (DOX) and glucose oxidase (Gox) for pancreatic cancer treatment. Int J Nanomed. 2023;18:7133–47.
Kumar R, Roy I, Ohulchanskky TY, Vathy LA, Bergey EJ, Sajjad M, et al. In vivo biodistribution and clearance studies using multimodal organically modified silica nanoparticles. ACS Nano. 2010;4(2):699–708.
Zhai W, He C, Wu L, Zhou Y, Chen H, Chang J, et al. Degradation of hollow mesoporous silica nanoparticles in human umbilical vein endothelial cells. J Biomed Mater Res B Appl Biomater. 2012;100B(5):1397–403.
Morelli C, Maris P, Sisci D, Perrotta E, Brunelli E, Perrotta I, et al. PEG-templated mesoporous silica nanoparticles exclusively target cancer cells. Nanoscale. 2011;3(8):3198–207.
Iversen T-G, Skotland T, Sandvig K. Endocytosis and intracellular transport of nanoparticles: present knowledge and need for future studies. Nano Today. 2011;6(2):176–85.
Huang X, Li L, Liu T, Hao N, Liu H, Chen D, et al. The shape effect of mesoporous silica nanoparticles on biodistribution, clearance, and biocompatibility in vivo. ACS Nano. 2011;5(7):5390–9.
Lu J, Liong M, Li Z, Zink JI, Tamanoi F. Biocompatibility, biodistribution, and drug-delivery efficiency of mesoporous silica nanoparticles for cancer therapy in animals. Small. 2010;6(16):1794–805.
Lu J, Li Z, Zink JI, Tamanoi F. In vivo tumor suppression efficacy of mesoporous silica nanoparticles-based drug-delivery system: enhanced efficacy by folate modification. Nanomed: Nanotechnol Biol Med. 2012;8(2):212–20.
Souris JS, Lee C-H, Cheng S-H, Chen C-T, Yang C-S, Ho J-AA, et al. Surface charge-mediated rapid hepatobiliary excretion of mesoporous silica nanoparticles. Biomaterials. 2010;31(21):5564–74.
Waegeneers N, Brasseur A, Van Doren E, Van der Heyden S, Serreyn P-J, Pussemier L, et al. Short-term biodistribution and clearance of intravenously administered silica nanoparticles. Toxicol Rep. 2018;5:632–8.
Croissant JG, Brinker CJ. Chapter Eight - Biodegradable silica-based nanoparticles: dissolution kinetics and selective bond cleavage. In: Tamanoi F, editor. The enzymes, vol. 43. Academic Press; 2018. p. 181–214.
Möller K, Bein T. Degradable drug carriers: vanishing mesoporous silica nanoparticles. Chem Mater. 2019;31(12):4364–78.
Cao Y, Li S, Chen J. Modeling better in vitro models for the prediction of nanoparticle toxicity: a review. Toxicol Mech Methods. 2021;31(1):1–17.
Lee K-I, Su C-C, Fang K-M, Wu C-C, Wu C-T, Chen Y-W. Ultrafine silicon dioxide nanoparticles cause lung epithelial cells apoptosis via oxidative stress-activated PI3K/Akt-mediated mitochondria- and endoplasmic reticulum stress-dependent signaling pathways. Sci Rep. 2020;10(1):9928.
Sun M, Zhang J, Liang S, Du Z, Liu J, Sun Z, et al. Metabolomic characteristics of hepatotoxicity in rats induced by silica nanoparticles. Ecotoxicol Environ Saf. 2021;208:111496.
Wang D-P, Wang Z-J, Zhao R, Lin C-X, Sun Q-Y, Yan C-P, et al. Silica nanomaterials induce organ injuries by Ca2+-ROS-initiated disruption of the endothelial barrier and triggering intravascular coagulation. Part Fibre Toxicol. 2020;17(1):12.
Hozayen WG, Mahmoud AM, Desouky EM, El-Nahass E-S, Soliman HA, Farghali AA. Cardiac and pulmonary toxicity of mesoporous silica nanoparticles is associated with excessive ROS production and redox imbalance in Wistar rats. Biomed Pharmacother. 2019;109:2527–38.
Yang X, Feng L, Zhang Y, Hu H, Shi Y, Liang S, et al. Co-exposure of silica nanoparticles and methylmercury induced cardiac toxicity in vitro and in vivo. Sci Total Environ. 2018;631–632:811–21.
Ma R, Qi Y, Zhao X, Li X, Sun X, Niu P, et al. Amorphous silica nanoparticles accelerated atherosclerotic lesion progression in ApoE−/− mice through endoplasmic reticulum stress-mediated CD36 up-regulation in macrophage. Part Fibre Toxicol. 2020;17(1):50.
Liu Y-Q, Xue S-M, Zhang P, Xu L-N, Wang D-P, Li G, et al. Silica nanoparticles disturb ion channels and transmembrane potentials of cardiomyocytes and induce lethal arrhythmias in mice. Int J Nanomedicine. 2020;15:7397–413.
Yu Y, Zhu T, Li Y, Jing L, Yang M, Li Y, et al. Repeated intravenous administration of silica nanoparticles induces pulmonary inflammation and collagen accumulation via JAK2/STAT3 and TGF-β/Smad3 pathways in vivo. Int J Nanomedicine. 2019;14:7237–47.
Parveen A, Rizvi SH, Sushma, Mahdi F, Ahmad I, Singh PP, et al. Intranasal exposure to silica nanoparticles induces alterations in pro-inflammatory environment of rat brain. Toxicol Ind Health. 2017;33(2):119–32.
Chou C-C, Chen W, Hung Y, Mou C-Y. Molecular elucidation of biological response to mesoporous silica nanoparticles in vitro and in vivo. ACS Appl Mater Interfaces. 2017;9(27):22235–51.
Liu Y, Wei H, Tang J, Yuan J, Wu M, Yao C, et al. Dysfunction of pulmonary epithelial tight junction induced by silicon dioxide nanoparticles via the ROS/ERK pathway and protein degradation. Chemosphere. 2020;255:126954.
Inoue M, Sakamoto K, Suzuki A, Nakai S, Ando A, Shiraki Y, et al. Size and surface modification of silica nanoparticles affect the severity of lung toxicity by modulating endosomal ROS generation in macrophages. Part Fibre Toxicol. 2021;18(1):1–20.
Zhang X, Luan J, Chen W, Fan J, Nan Y, Wang Y, et al. Mesoporous silica nanoparticles induced hepatotoxicity via NLRP3 inflammasome activation and caspase-1-dependent pyroptosis. Nanoscale. 2018;10(19):9141–52.
Magaye RR, Yue X, Zou B, Shi H, Yu H, Liu K, et al. Acute toxicity of nickel nanoparticles in rats after intravenous injection. Int J Nanomedicine. 2014;9:1393–402.
Guo C, Liu Y, Li Y. Adverse effects of amorphous silica nanoparticles: focus on human cardiovascular health. J Hazard Mater. 2021;406:124626.
Aouey B, Boukholda K, Gargouri B, Bhatia HS, Attaai A, Kebieche M, et al. Silica nanoparticles induce hepatotoxicity by triggering oxidative damage, apoptosis, and Bax-Bcl2 signaling pathway. Biol Trace Elem Res. 2021;200(4):1688–98.
Lozano O, Silva-Platas C, Chapoy-Villanueva H, Pérez BE, Lees JG, Ramachandra CJA, et al. Amorphous SiO2 nanoparticles promote cardiac dysfunction via the opening of the mitochondrial permeability transition pore in rat heart and human cardiomyocytes. Part Fibre Toxicol. 2020;17(1):15.
Deng Y-D, Zhang X-D, Yang X-S, Huang Z-L, Wei X, Yang X-F, et al. Subacute toxicity of mesoporous silica nanoparticles to the intestinal tract and the underlying mechanism. J Hazard Mater. 2021;409:124502.
Berry JP, Arnoux B, Stanislas G, Galle P, Chretien J. A microanalytic study of particles transport across the alveoli: role of blood platelets. Biomedicine / [publiee pour l’AAICIG]. 1977;27(9–10):354–7.
Nemmar A, Hoet PH, Vanquickenborne B, Dinsdale D, Thomeer M, Hoylaerts MF, et al. Passage of inhaled particles into the blood circulation in humans. Circulation. 2002;105(4):411–4.
Oberdörster G, Oberdörster E, Oberdörster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113(7):823–39.
Mohamed IN, Sheibani N, El-Remessy AB. Deletion of thioredoxin-interacting protein (TXNIP) abrogates high fat diet-induced retinal leukostasis, barrier dysfunction and microvascular degeneration in a mouse obesity model. Int J Mol Sci. 2020;21(11):3983.
Wang M, Li J, Dong S, Cai X, Simaiti A, Yang X, et al. Silica nanoparticles induce lung inflammation in mice via ROS/PARP/TRPM2 signaling-mediated lysosome impairment and autophagy dysfunction. Part Fibre Toxicol. 2020;17(1):23.
Van Rijt SH, Bölükbas DA, Argyo C, Wipplinger K, Naureen M, Datz S, et al. Applicability of avidin protein coated mesoporous silica nanoparticles as drug carriers in the lung. Nanoscale. 2016;8(15):8058–69.
Wottrich R, Diabaté S, Krug HF. Biological effects of ultrafine model particles in human macrophages and epithelial cells in mono-and co-culture. Int J Hyg Environ Health. 2004;207(4):353–61.
Mohammadpour R, Yazdimamaghani M, Cheney DL, Jedrzkiewicz J, Ghandehari H. Subchronic toxicity of silica nanoparticles as a function of size and porosity. J Control Release. 2019;304:216–32.
Duan J, Yu Y, Li Y, Yu Y, Li Y, Zhou X, et al. Toxic effect of silica nanoparticles on endothelial cells through DNA damage response via Chk1-dependent G2/M checkpoint. PLoS ONE. 2013;8(4):e62087.
Bauer AT, Strozyk EA, Gorzelanny C, Westerhausen C, Desch A, Schneider MF, et al. Cytotoxicity of silica nanoparticles through exocytosis of von Willebrand factor and necrotic cell death in primary human endothelial cells. Biomaterials. 2011;32(33):8385–93.
Yazdimamaghani M, Moos PJ, Dobrovolskaia MA, Ghandehari H. Genotoxicity of amorphous silica nanoparticles: status and prospects. Nanomed Nanotechnol Biol Med. 2019;16:106–25.
Kharlamov AN, Feinstein JA, Cramer JA, Boothroyd JA, Shishkina EV, Shur V. Plasmonic photothermal therapy of atherosclerosis with nanoparticles: long-term outcomes and safety in NANOM-FIM trial. Future Cardiol. 2017;13(4):345–63.
Rastinehad AR, Anastos H, Wajswol E, Winoker JS, Sfakianos JP, Doppalapudi SK, et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc Natl Acad Sci. 2019;116(37):18590–6.
Phillips E, Penate-Medina O, Zanzonico PB, Carvajal RD, Mohan P, Ye Y, et al. Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe. Sci Transl Med. 2014;6(260):260ra149.
Zanoni DK, Stambuk HE, Madajewski B, Montero PH, Matsuura D, Busam KJ, et al. Use of ultrasmall core-shell fluorescent silica nanoparticles for image-guided sentinel lymph node biopsy in head and neck melanoma: a nonrandomized clinical trial. JAMA Netw Open. 2021;4(3):e211936-e.
Hargrove D, Liang B, Kashfi-Sadabad R, Joshi GN, Gonzalez-Fajardo L, Glass S, et al. Tumor-mesoporous silica nanoparticle interactions following intraperitoneal delivery for targeting peritoneal metastasis. J Control Release: Off J Control Release Soc. 2020;328:846–58.
Di Pasqua AJ, Yuan H, Chung Y, Kim JK, Huckle JE, Li C, et al. Neutron-activatable holmium-containing mesoporous silica nanoparticles as a potential radionuclide therapeutic agent for ovarian cancer. J Nucl Med: Off Publ Soc Nucl Med. 2013;54(1):111–6.
Acknowledgements
The authors would like to acknowledge Dr. Derek Hargrove for his valuable insights on the manuscript.
Funding
We appreciate the financial support from the National Cancer Institute of the National Institutes of Health R41CA239989, R44CA239989, and the Research Scholar Grant RSG-15–011-01-CDD from the American Cancer Society.
Author information
Authors and Affiliations
Contributions
Joshua Yu: conceptualization, literature review, and writing—original draft. Nirnoy Dan: conceptualization, literature review, and writing—original draft discussion. Seyyed Majid Eslami: writing—review and editing. Xiuling Lu: conceptualization, supervision, and writing—review and editing.
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Xiuling Lu is an inventor of intellectual property licensed to Nami Therapeutics Corp for developing silica nanoparticle-based radiopharmaceuticals. Dr. Xiuling Lu owns equity. It is important to note that the analysis and interpretation in this review were without any influence from the potential financial gains. The authors affirm that the conclusions drawn and the information provided in this paper are based on scientific evidence.
Additional information
Communicated by Aliasger Salem
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, J., Dan, N., Eslami, S.M. et al. State of the Art of Silica Nanoparticles: An Overview on Biodistribution and Preclinical Toxicity Studies. AAPS J 26, 35 (2024). https://doi.org/10.1208/s12248-024-00906-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-024-00906-w