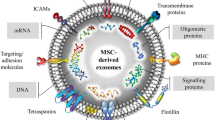
Abstract
Traumatic brain injury (TBI) of all severities is a significant public health burden, causing a range of effects that can lead to death or a diminished quality of life. Liposomes and mesenchymal stem cell-derived exosomes are two drug delivery agents with potential to be leveraged in the treatment of TBI by increasing the efficacy of drug therapies as well as having additional therapeutic effects. They exhibit several physical similarities, but key differences affect their performances as nanocarriers. Liposomes can be produced commercially at scale, and liposomes achieve higher encapsulation efficiency. Meanwhile, the intrinsic cargo and targeting moieties of exosomes, which liposomes lack, give exosomes a greater ability to facilitate neural regeneration, and exosomes do not trigger the infusion reactions that liposomes can. However, there are concerns about both exosomes and liposomes regarding interactions with tumors. The same routes of administration can be used for both exosomes and liposomes, resulting in somewhat different distribution throughout the body. While the effect of the nanocarrier type on accumulation in the brain is not concrete, targeting leads to increased accumulation of both exosomes and liposomes in the brain, upon which on-demand release can be used for both drug deliverers. Although neither have been applied to TBI in humans, preclinical trials have shown their immense potential, as have clinical trials pertaining to other brain injuries and conditions. While questions remain, research thus far shows that the various differences make exosomes a better choice of nanocarrier for TBI.
Graphical Abstract


Similar content being viewed by others
References
Georges AJ MD. Traumatic brain injury. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC. 2022.
Rakhit S, Nordness MF, Lombardo SR, Cook M, Smith L, Patel MB. Management and challenges of severe traumatic brain injury. Semin Respir Crit Care Med. 2021;42(1):127–44.
Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic brain injury-related emergency department visits, hospitalizations, and deaths - United States, 2007 and 2013. MMWR Surveill Summ. 2017;66(9):1–16.
Lim SH, Jung H, Youn DH, Kim TY, Han SW, Kim BJ, et al. Mild traumatic brain injury and subsequent acute pulmonary inflammatory response. J Korean Neurosurg Soc. 2022;65(5):680–7.
Dixon KJ. Pathophysiology of traumatic brain injury. Phys Med Rehabil Clin N Am. 2017;28(2):215–25.
Laskowski RA, Creed JA, Raghupathi R. Pathophysiology of mild TBI: implications for altered signaling pathways. In: Kobeissy FH, editor. Brain neurotrauma: molecular, neuropsychological, and rehabilitation aspects. Frontiers in Neuroengineering. Boca Raton (FL): CRC Press/Taylor & Francis © 2015 by Taylor & Francis Group, LLC. 2015.
McCrea MA, Giacino JT, Barber J, Temkin NR, Nelson LD, Levin HS, et al. Functional outcomes over the first year after moderate to severe traumatic brain injury in the prospective, longitudinal TRACK-TBI study. JAMA Neurol. 2021;78(8):982–92.
Ma X, Cheng Y, Garcia R, Haorah J. Hemorrhage associated mechanisms of neuroinflammation in experimental traumatic brain injury. J Neuroimmune Pharmacol. 2020;15(2):181–95.
Simon DW, McGeachy MJ, Bayır H, Clark RS, Loane DJ, Kochanek PM. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat Rev Neurol. 2017;13(3):171–91.
Karve IP, Taylor JM, Crack PJ. The contribution of astrocytes and microglia to traumatic brain injury. Br J Pharmacol. 2016;173(4):692–702.
Hay JR, Johnson VE, Young AM, Smith DH, Stewart W. Blood-brain barrier disruption is an early event that may persist for many years after traumatic brain injury in humans. J Neuropathol Exp Neurol. 2015;74(12):1147–57.
Nasr IW, Chun Y, Kannan S. Neuroimmune responses in the developing brain following traumatic brain injury. Exp Neurol. 2019;320: 112957.
Zhu H, Wang Z, Yu J, Yang X, He F, Liu Z, et al. Role and mechanisms of cytokines in the secondary brain injury after intracerebral hemorrhage. Prog Neurobiol. 2019;178: 101610.
Takahashi T, Marushima A, Nagasaki Y, Hirayama A, Muroi A, Puentes S, et al. Novel neuroprotection using antioxidant nanoparticles in a mouse model of head trauma. J Trauma Acute Care Surg. 2020;88(5):677–85.
Scarboro M, McQuillan KA. Traumatic brain injury update. AACN Adv Crit Care. 2021;32(1):29–50.
Vella MA, Crandall ML, Patel MB. Acute management of traumatic brain injury. Surg Clin North Am. 2017;97(5):1015–30.
Loane DJ, Stoica BA, Faden AI. Neuroprotection for traumatic brain injury. Handb Clin Neurol. 2015;127:343–66.
Xiong Y, Mahmood A, Chopp M. Neurorestorative treatments for traumatic brain injury. Discov Med. 2010;10(54):434–42.
Ghiam MK, Patel SD, Hoffer A, Selman WR, Hoffer BJ, Hoffer ME. Drug repurposing in the treatment of traumatic brain injury. Front Neurosci. 2021;15: 635483.
Xu D, Wu D, Qin M, Nih LR, Liu C, Cao Z, et al. Efficient Delivery of Nerve Growth Factors to the Central Nervous System for Neural Regeneration. Adv Mater. 2019;31(33): e1900727.
Adugna DG, Aragie H, Kibret AA, Belay DG. Therapeutic Application of stem cells in the repair of traumatic brain injury. Stem Cells Cloning. 2022;15:53–61.
Omidi Y, Barar J. Impacts of blood-brain barrier in drug delivery and targeting of brain tumors. Bioimpacts. 2012;2(1):5–22.
Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol. 2015;7(1): a020412.
Zhao Y, Gan L, Ren L, Lin Y, Ma C, Lin X. Factors influencing the blood-brain barrier permeability. Brain Res. 2022;1788: 147937.
Cash A, Theus MH. Mechanisms of blood-brain barrier dysfunction in traumatic brain injury. Int J Mol Sci. 2020;21(9).
Lai RC, Yeo RW, Lim SK. Mesenchymal stem cell exosomes. Semin Cell Dev Biol. 2015;40:82–8.
Willms E, Johansson HJ, Mager I, Lee Y, Blomberg KE, Sadik M, et al. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci Rep. 2016;6:22519.
Gurung S, Perocheau D, Touramanidou L, Baruteau J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun Signal. 2021;19(1):47.
Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, et al. Liposome: classification, preparation, and applications. Nanoscale Res Lett. 2013;8(1):102.
Vieira DB, Gamarra LF. Getting into the brain: liposome-based strategies for effective drug delivery across the blood-brain barrier. Int J Nanomedicine. 2016;11:5381–414.
Szebeni J, Baranyi L, Savay S, Bodo M, Morse DS, Basta M, et al. Liposome-induced pulmonary hypertension: properties and mechanism of a complement-mediated pseudoallergic reaction. Am J Physiol Heart Circ Physiol. 2000;279(3):H1319–28.
Ruozi B, Belletti D, Tombesi A, Tosi G, Bondioli L, Forni F, et al. AFM, ESEM, TEM, and CLSM in liposomal characterization: a comparative study. Int J Nanomedicine. 2011;6:557–63.
Aqil F, Munagala R, Jeyabalan J, Agrawal AK, Gupta R. Exosomes for the enhanced tissue bioavailability and efficacy of curcumin. AAPS J. 2017;19(6):1691–702.
Sonali Singh RP, Singh N, Sharma G, Vijayakumar MR, Koch B, et al. Transferrin liposomes of docetaxel for brain-targeted cancer applications: formulation and brain theranostics. Drug Deliv. 2016;23(4):1261–71.
Kimiz-Gebologlu I, Oncel SS. Exosomes: large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J Control Release. 2022;347:533–43.
Yeo RW, Lai RC, Zhang B, Tan SS, Yin Y, Teh BJ, et al. Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev. 2013;65(3):336–41.
Syromiatnikova V, Prokopeva A, Gomzikova M. Methods of the large-scale production of extracellular vesicles. Int J Mol Sci. 2022;23(18).
Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in exosome isolation techniques. Theranostics. 2017;7(3):789–804.
Chen J, Li P, Zhang T, Xu Z, Huang X, Wang R, et al. Review on strategies and technologies for exosome isolation and purification. Front Bioeng Biotechnol. 2021;9:811971.
Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, et al. Reassessment of exosome composition. Cell. 2019;177(2):428-45.e18.
Dhar R, Gorai S, Devi A, Muthusamy R, Alexiou A, Papadakis M. Decoding of exosome heterogeneity for cancer theranostics. Clin Transl Med. 2023;13(6): e1288.
Mashouri L, Yousefi H, Aref AR, Ahadi AM, Molaei F, Alahari SK. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer. 2019;18(1):75.
Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019;88:487–514.
Lai JJ, Chau ZL, Chen SY, Hill JJ, Korpany KV, Liang NW, et al. Exosome processing and characterization approaches for research and technology development. Adv Sci (Weinheim, Baden-Wurttemberg, Germany). 2022;9(15):e2103222.
Helwa I, Cai J, Drewry MD, Zimmerman A, Dinkins MB, Khaled ML, et al. A comparative study of serum exosome isolation using differential ultracentrifugation and three commercial reagents. PLoS ONE. 2017;12(1): e0170628.
Chen YS, Lin EY, Chiou TW, Harn HJ. Exosomes in clinical trial and their production in compliance with good manufacturing practice. Ci Ji Yi Xue Za Zhi. 2020;32(2):113–20.
Ahn SH, Ryu SW, Choi H, You S, Park J, Choi C. Manufacturing therapeutic exosomes: from bench to industry. Mol Cells. 2022;45(5):284–90.
Maumus M, Rozier P, Boulestreau J, Jorgensen C, Noel D. Mesenchymal stem cell-derived extracellular vesicles: opportunities and challenges for clinical translation. Front Bioeng Biotechnol. 2020;8:997.
Shah S, Dhawan V, Holm R, Nagarsenker MS, Perrie Y. Liposomes: advancements and innovation in the manufacturing process. Adv Drug Deliv Rev. 2020;154–155:102–22.
Worsham RD, Thomas V, Farid SS. Potential of continuous manufacturing for liposomal drug products. Biotechnol J. 2019;14(2): e1700740.
Charcosset C, Juban A, Valour J-P, Urbaniak S, Fessi H. Preparation of liposomes at large scale using the ethanol injection method: effect of scale-up and injection devices. Chem Eng Res Des. 2015;94:508–15.
Carugo D, Bottaro E, Owen J, Stride E, Nastruzzi C. Liposome production by microfluidics: potential and limiting factors. Sci Rep. 2016;6:25876.
Forbes N, Hussain MT, Briuglia ML, Edwards DP, Horst JHT, Szita N, et al. Rapid and scale-independent microfluidic manufacture of liposomes entrapping protein incorporating in-line purification and at-line size monitoring. Int J Pharm. 2019;556:68–81.
Sydykov B, Oldenhof H, Sieme H, Wolkers WF. Storage stability of liposomes stored at elevated subzero temperatures in DMSO/sucrose mixtures. PLoS ONE. 2018;13(7): e0199867.
Muppidi K, Pumerantz AS, Wang J, Betageri G. Development and stability studies of novel liposomal vancomycin formulations. ISRN Pharm. 2012;2012: 636743.
Shashidhar GM, Manohar B. Nanocharacterization of liposomes for the encapsulation of water soluble compounds from Cordyceps sinensis CS1197 by a supercritical gas anti-solvent technique. RSC Adv. 2018;8(60):34634–49.
Doi Y, Shimizu T, Ishima Y, Ishida T. Long-term storage of PEGylated liposomal oxaliplatin with improved stability and long circulation times in vivo. Int J Pharm. 2019;564:237–43.
Cern A, Marcus D, Tropsha A, Barenholz Y, Goldblum A. New drug candidates for liposomal delivery identified by computer modeling of liposomes’ remote loading and leakage. J Control Release. 2017;252:18–27.
Jeyaram A, Jay SM. Preservation and storage stability of extracellular vesicles for therapeutic applications. AAPS J. 2017;20(1):1.
Xiong Y, Mahmood A, Chopp M. Emerging potential of exosomes for treatment of traumatic brain injury. Neural Regen Res. 2017;12(1):19–22.
Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X, et al. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells. 2012;30(7):1556–64.
Lai RC, Tan SS, Teh BJ, Sze SK, Arslan F, de Kleijn DP, et al. Proteolytic potential of the MSC exosome proteome: implications for an exosome-mediated delivery of therapeutic proteasome. Int J Proteomics. 2012;2012: 971907.
Deng W, Chen W, Clement S, Guller A, Zhao Z, Engel A, et al. Controlled gene and drug release from a liposomal delivery platform triggered by X-ray radiation. Nat Commun. 2018;9(1):2713.
Ni H, Yang S, Siaw-Debrah F, Hu J, Wu K, He Z, et al. Exosomes derived from bone mesenchymal stem cells ameliorate early inflammatory responses following traumatic brain injury. Front Neurosci. 2019;13:14.
Zhang Y, Chopp M, Meng Y, Katakowski M, Xin H, Mahmood A, et al. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg. 2015;122(4):856–67.
Tajiri N, Acosta SA, Shahaduzzaman M, Ishikawa H, Shinozuka K, Pabon M, et al. Intravenous transplants of human adipose-derived stem cell protect the brain from traumatic brain injury-induced neurodegeneration and motor and cognitive impairments: cell graft biodistribution and soluble factors in young and aged rats. J Neurosci. 2014;34(1):313–26.
Popa-Wagner A, Buga AM, Doeppner TR, Hermann DM. Stem cell therapies in preclinical models of stroke associated with aging. Front Cell Neurosci. 2014;8:347.
Javidi E, Magnus T. Autoimmunity after ischemic stroke and brain injury. Front Immunol. 2019;10:686.
Zhang Y, Chopp M, Zhang ZG, Katakowski M, Xin H, Qu C, et al. Systemic administration of cell-free exosomes generated by human bone marrow derived mesenchymal stem cells cultured under 2D and 3D conditions improves functional recovery in rats after traumatic brain injury. Neurochem Int. 2017;111:69–81.
Sharma S, Ifergan I, Kurz JE, Linsenmeier RA, Xu D, Cooper JG, et al. Intravenous immunomodulatory nanoparticle treatment for traumatic brain injury. Ann Neurol. 2020;87(3):442–55.
Rajan R, Sabnani MK, Mavinkurve V, Shmeeda H, Mansouri H, Bonkoungou S, et al. Liposome-induced immunosuppression and tumor growth is mediated by macrophages and mitigated by liposome-encapsulated alendronate. J Control Release. 2018;271:139–48.
Pervin M, Golbar HM, Bondoc A, Izawa T, Kuwamura M, Yamate J. Transient effects of empty liposomes on hepatic macrophage populations in rats. J Toxicol Pathol. 2016;29(2):139–44.
Begemann M, Leon M, van der Horn HJ, van der Naalt J, Sommer I. Drugs with anti-inflammatory effects to improve outcome of traumatic brain injury: a meta-analysis. Sci Rep. 2020;10(1):16179.
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341–5.
Ahmed S, Salmon H, Distasio N, Do HD, Scherman D, Alhareth K, et al. Viscous core liposomes increase siRNA encapsulation and provides gene inhibition when slightly positively charged. Pharmaceutics. 2021;13(4).
Lombardo D, Kiselev MA. Methods of liposomes preparation: formation and control factors of versatile nanocarriers for biomedical and nanomedicine application. Pharmaceutics. 2022;14(3).
Hardiansyah A, Yang MC, Liu TY, Kuo CY, Huang LY, Chan TY. Hydrophobic drug-loaded PEGylated magnetic liposomes for drug-controlled release. Nanoscale Res Lett. 2017;12(1):355.
Fu S, Wang Y, Xia X, Zheng JC. Exosome engineering: current progress in cargo loading and targeted delivery. NanoImpact. 2020;20: 100261.
Fuhrmann G, Serio A, Mazo M, Nair R, Stevens MM. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J Control Release. 2015;205:35–44.
Mehryab F, Rabbani S, Shahhosseini S, Shekari F, Fatahi Y, Baharvand H, et al. Exosomes as a next-generation drug delivery system: an update on drug loading approaches, characterization, and clinical application challenges. Acta Biomater. 2020;113:42–62.
Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine. 2016;12(3):655–64.
Xi XM, Xia SJ, Lu R. Drug loading techniques for exosome-based drug delivery systems. Pharmazie. 2021;76(2):61–7.
Jeyaram A, Lamichhane TN, Wang S, Zou L, Dahal E, Kronstadt SM, et al. Enhanced loading of functional miRNA cargo via pH gradient modification of extracellular vesicles. Mol Ther. 2020;28(3):975–85.
Liu H, Shen M, Zhao D, Ru D, Duan Y, Ding C, et al. The effect of triptolide-loaded exosomes on the proliferation and apoptosis of human ovarian cancer SKOV3 cells. Biomed Res Int. 2019;2019:2595801.
Fritze A, Hens F, Kimpfler A, Schubert R, Peschka-Süss R. Remote loading of doxorubicin into liposomes driven by a transmembrane phosphate gradient. Biochim Biophys Acta. 2006;1758(10):1633–40.
Tran PHL, Wang T, Yin W, Tran TTD, Barua HT, Zhang Y, et al. Development of a nanoamorphous exosomal delivery system as an effective biological platform for improved encapsulation of hydrophobic drugs. Int J Pharm. 2019;566:697–707.
Hong SS, Kim SH, Lim SJ. Effects of triglycerides on the hydrophobic drug loading capacity of saturated phosphatidylcholine-based liposomes. Int J Pharm. 2015;483(1–2):142–50.
Chaves MA, Oseliero Filho PL, Jange CG, Sinigaglia-Coimbra R, Oliveira CLP, Pinho SC. Structural characterization of multilamellar liposomes coencapsulating curcumin and vitamin D3. Colloids Surf, A. 2018;549:112–21.
Pouw RB, Ricklin D. Tipping the balance: intricate roles of the complement system in disease and therapy. Semin Immunopathol. 2021;43(6):757–71.
Goetzl EJ, Yaffe K, Peltz CB, Ledreux A, Gorgens K, Davidson B, et al. Traumatic brain injury increases plasma astrocyte-derived exosome levels of neurotoxic complement proteins. FASEB J. 2020;34(2):3359–66.
Winston CN, Romero HK, Ellisman M, Nauss S, Julovich DA, Conger T, et al. Assessing neuronal and astrocyte derived exosomes from individuals with mild traumatic brain injury for markers of neurodegeneration and cytotoxic activity. Front Neurosci. 2019;13:1005.
Ferreira LC, Regner A, Miotto KD, Moura S, Ikuta N, Vargas AE, et al. Increased levels of interleukin-6, -8 and -10 are associated with fatal outcome following severe traumatic brain injury. Brain Inj. 2014;28(10):1311–6.
Guedes VA, Devoto C, Leete J, Sass D, Acott JD, Mithani S, et al. Extracellular vesicle proteins and microRNAs as biomarkers for traumatic brain injury. Front Neurol. 2020;11:663.
Milosevits G, Szebeni J, Krol S. Exosomes: potential model for complement-stealth delivery systems. Eur J Nanomedicine. 2015;7(3):207–18.
Williams AM, Dennahy IS, Bhatti UF, Halaweish I, Xiong Y, Chang P, et al. Mesenchymal stem cell-derived exosomes provide neuroprotection and improve long-term neurologic outcomes in a swine model of traumatic brain injury and hemorrhagic shock. J Neurotrauma. 2019;36(1):54–60.
Loh JT, Zhang B, Teo JKH, Lai RC, Choo ABH, Lam KP, et al. Mechanism for the attenuation of neutrophil and complement hyperactivity by MSC exosomes. Cytotherapy. 2022;24(7):711–9.
Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Advances and challenges of liposome assisted drug delivery. Front Pharmacol. 2015;6:286.
Milla P, Dosio F, Cattel L. PEGylation of proteins and liposomes: a powerful and flexible strategy to improve the drug delivery. Curr Drug Metab. 2012;13(1):105–19.
Fulop T, Kozma GT, Vashegyi I, Meszaros T, Rosivall L, Urbanics R, et al. Liposome-induced hypersensitivity reactions: risk reduction by design of safe infusion protocols in pigs. J Control Release. 2019;309:333–8.
Inglut CT, Sorrin AJ, Kuruppu T, Vig S, Cicalo J, Ahmad H, et al. Immunological and toxicological considerations for the design of liposomes. Nanomaterials (Basel). 2020;10(2).
Yang Q, Jacobs TM, McCallen JD, Moore DT, Huckaby JT, Edelstein JN, et al. Analysis of pre-existing IgG and IgM antibodies against polyethylene glycol (PEG) in the general population. Anal Chem. 2016;88(23):11804–12.
Szebeni J, Bedocs P, Urbanics R, Bunger R, Rosivall L, Toth M, et al. Prevention of infusion reactions to PEGylated liposomal doxorubicin via tachyphylaxis induction by placebo vesicles: a porcine model. J Control Release. 2012;160(2):382–7.
McSweeney MD, Price LSL, Wessler T, Ciociola EC, Herity LB, Piscitelli JA, et al. Overcoming anti-PEG antibody mediated accelerated blood clearance of PEGylated liposomes by pre-infusion with high molecular weight free PEG. J Control Release. 2019;311–312:138–46.
Mohamadpour M, Whitney K, Bergold PJ. The importance of therapeutic time window in the treatment of traumatic brain injury. Front Neurosci. 2019;13:07.
Kumar V, Qin J, Jiang Y, Duncan RG, Brigham B, Fishman S, et al. Shielding of lipid nanoparticles for siRNA delivery: impact on physicochemical properties, cytokine induction, and efficacy. Mol Ther Nucleic Acids. 2014;3(11): e210.
Maisha N, Naik N, Okesola M, Coombs T, Zilberberg R, Pandala N, et al. Engineering PEGylated polyester nanoparticles to reduce complement-mediated infusion reaction. Bioconjug Chem. 2021;32(10):2154–66.
Shin K, Suh HW, Grundler J, Lynn AY, Pothupitiya JU, Moscato ZM, et al. Polyglycerol and poly(ethylene glycol) exhibit different effects on pharmacokinetics and antibody generation when grafted to nanoparticle surfaces. Biomaterials. 2022;287: 121676.
Nag OK, Yadav VR, Croft B, Hedrick A, Awasthi V. Liposomes modified with superhydrophilic polymer linked to a nonphospholipid anchor exhibit reduced complement activation and enhanced circulation. J Pharm Sci. 2015;104(1):114–23.
Montizaan D, Yang K, Reker-Smit C, Salvati A. Comparison of the uptake mechanisms of zwitterionic and negatively charged liposomes by HeLa cells. Nanomedicine. 2020;30: 102300.
Wang Z, Hood ED, Nong J, Ding J, Marcos-Contreras OA, Glassman PM, et al. Combating complement’s deleterious effects on nanomedicine by conjugating complement regulatory proteins to nanoparticles. Adv Mater. 2022;34(8): e2107070.
Parodi A, Quattrocchi N, van de Ven AL, Chiappini C, Evangelopoulos M, Martinez JO, et al. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat Nanotechnol. 2013;8(1):61–8.
Yang Y, Bucan V, Baehre H, von der Ohe J, Otte A, Hass R. Acquisition of new tumor cell properties by MSC-derived exosomes. Int J Oncol. 2015;47(1):244–52.
Li H, Li F. Exosomes from BM-MSCs increase the population of CSCs via transfer of miR-142-3p. Br J Cancer. 2018;119(6):744–55.
Ono M, Kosaka N, Tominaga N, Yoshioka Y, Takeshita F, Takahashi RU, et al. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci Signal. 2014;7(332):ra63.
Konig S, Regen T, Dittmann K, Engelke M, Wienands J, Schwendener R, et al. Empty liposomes induce antitumoral effects associated with macrophage responses distinct from those of the TLR1/2 agonist Pam3CSK 4 (BLP). Cancer Immunol Immunother. 2013;62(10):1587–97.
Ioannides P, Giedzinski E, Limoli CL. Evaluating different routes of extracellular vesicle administration for cranial therapies. J Cancer Metastasis Treat. 2020;6(15).
Saka R, Chella N, Khan W. Development of imatinib mesylate-loaded liposomes for nose to brain delivery: in vitro and in vivo evaluation. AAPS PharmSciTech. 2021;22(5):192.
Ye Z, Gastfriend BD, Umlauf BJ, Lynn DM, Shusta EV. Antibody-targeted liposomes for enhanced targeting of the blood-brain barrier. Pharm Res. 2022;39(7):1523–34.
Duong VA, Nguyen TT, Maeng HJ. Recent advances in intranasal liposomes for drug, gene, and vaccine delivery. Pharmaceutics. 2023;15(1).
Betzer O, Perets N, Angel A, Motiei M, Sadan T, Yadid G, et al. In vivo neuroimaging of exosomes using gold nanoparticles. ACS Nano. 2017;11(11):10883–93.
Wiklander OP, Nordin JZ, O’Loughlin A, Gustafsson Y, Corso G, Mager I, et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles. 2015;4:26316.
Mirzaaghasi A, Han Y, Ahn SH, Choi C, Park JH. Biodistribution and pharmacokinectics of liposomes and exosomes in a mouse model of sepsis. Pharmaceutics. 2021;13(3).
Hood JL, Wickline SA. A systematic approach to exosome-based translational nanomedicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012;4(4):458–67.
Liang Y, Duan L, Lu J, Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11(7):3183–95.
Choi H, Choi Y, Yim HY, Mirzaaghasi A, Yoo JK, Choi C. Biodistribution of exosomes and engineering strategies for targeted delivery of therapeutic exosomes. Tissue Eng Regen Med. 2021;18(4):499–511.
Almeida B, Nag OK, Rogers KE, Delehanty JB. Recent progress in bioconjugation strategies for liposome-mediated drug delivery. Molecules. 2020;25(23).
Yassin MA, Appelhans D, Wiedemuth R, Formanek P, Boye S, Lederer A, et al. Overcoming concealment effects of targeting moieties in the PEG corona: controlled permeable polymersomes decorated with folate-antennae for selective targeting of tumor cells. Small. 2015;11(13):1580–91.
Heidarzadeh M, Gursoy-Ozdemir Y, Kaya M, Eslami Abriz A, Zarebkohan A, Rahbarghazi R, et al. Exosomal delivery of therapeutic modulators through the blood-brain barrier; promise and pitfalls. Cell Biosci. 2021;11(1):142.
Cantres-Rosario YM, Wojna V, Ruiz R, Diaz B, Matos M, Rodriguez-Benitez RJ, et al. Soluble insulin receptor levels in plasma, exosomes, and urine and its association with HIV-associated neurocognitive disorders. Front Neurol. 2022;13: 809956.
Marcos-Contreras OA, Greineder CF, Kiseleva RY, Parhiz H, Walsh LR, Zuluaga-Ramirez V, et al. Selective targeting of nanomedicine to inflamed cerebral vasculature to enhance the blood-brain barrier. Proc Natl Acad Sci U S A. 2020;117(7):3405–14.
Zhang Y, Boado RJ, Pardridge WM. Marked enhancement in gene expression by targeting the human insulin receptor. J Gene Med. 2003;5(2):157–63.
Pinzon-Daza M, Garzon R, Couraud P, Romero I, Weksler B, Ghigo D, et al. The association of statins plus LDL receptor-targeted liposome-encapsulated doxorubicin increases in vitro drug delivery across blood-brain barrier cells. Br J Pharmacol. 2012;167(7):1431–47.
Fu C, Xiang Y, Li X, Fu A. Targeted transport of nanocarriers into brain for theranosis with rabies virus glycoprotein-derived peptide. Mater Sci Eng, C. 2018;87:155–66.
Cui GH, Guo HD, Li H, Zhai Y, Gong ZB, Wu J, et al. RVG-modified exosomes derived from mesenchymal stem cells rescue memory deficits by regulating inflammatory responses in a mouse model of Alzheimer’s disease. Immun Ageing. 2019;16:10.
Xin X, Liu W, Zhang ZA, Han Y, Qi LL, Zhang YY, et al. Efficient anti-glioma therapy through the brain-targeted RVG15-modified liposomes loading paclitaxel-cholesterol complex. Int J Nanomedicine. 2021;16:5755–76.
Patel NA, Moss LD, Lee JY, Tajiri N, Acosta S, Hudson C, et al. Long noncoding RNA MALAT1 in exosomes drives regenerative function and modulates inflammation-linked networks following traumatic brain injury. J Neuroinflammation. 2018;15(1):204.
Wang Z, Zhao Y, Jiang Y, Lv W, Wu L, Wang B, et al. Enhanced anti-ischemic stroke of ZL006 by T7-conjugated PEGylated liposomes drug delivery system. Sci Rep. 2015;5:12651.
Qu M, Lin Q, Huang L, Fu Y, Wang L, He S, et al. Dopamine-loaded blood exosomes targeted to brain for better treatment of Parkinson’s disease. J Control Release. 2018;287:156–66.
Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478).
Li G, Wang J, Xu M, Zhang H, Tu C, Yang J, et al. Engineered exosome for NIR-triggered drug delivery and superior synergistic chemo-phototherapy in a glioma model. Appl Mater Today. 2020;20: 100723.
Russell LM, Hultz M, Searson PC. Leakage kinetics of the liposomal chemotherapeutic agent Doxil: the role of dissolution, protonation, and passive transport, and implications for mechanism of action. J Control Release. 2018;269:171–6.
Yang J, Bahreman A, Daudey G, Bussmann J, Olsthoorn RC, Kros A. Drug delivery via cell membrane fusion using lipopeptide modified liposomes. ACS Cent Sci. 2016;2(9):621–30.
Farid M, Faber T, Dietrich D, Lamprecht A. Cell membrane fusing liposomes for cytoplasmic delivery in brain endothelial cells. Colloids Surf B Biointerfaces. 2020;194: 111193.
Yang Y, Liu X, Ma W, Xu Q, Chen G, Wang Y, et al. Light-activatable liposomes for repetitive on-demand drug release and immunopotentiation in hypoxic tumor therapy. Biomaterials. 2021;265: 120456.
Sirsi SR, Borden MA. State-of-the-art materials for ultrasound-triggered drug delivery. Adv Drug Deliv Rev. 2014;72:3–14.
de Matos MBC, Beztsinna N, Heyder C, Fens M, Mastrobattista E, Schiffelers RM, et al. Thermosensitive liposomes for triggered release of cytotoxic proteins. Eur J Pharm Biopharm. 2018;132:211–21.
Dromi S, Frenkel V, Luk A, Traughber B, Angstadt M, Bur M, et al. Pulsed-high intensity focused ultrasound and low temperature-sensitive liposomes for enhanced targeted drug delivery and antitumor effect. Clin Cancer Res. 2007;13(9):2722–7.
Schroeder A, Avnir Y, Weisman S, Najajreh Y, Gabizon A, Talmon Y, et al. Controlling liposomal drug release with low frequency ultrasound: mechanism and feasibility. Langmuir. 2007;23(7):4019–25.
Bulbake U, Doppalapudi S, Kommineni N, Khan W. Liposomal formulations in clinical use: an updated review. Pharmaceutics. 2017;9(2).
Dehghani L, Khojasteh A, Soleimani M, Oraee-Yazdani S, Keshel SH, Saadatnia M, et al. Safety of intraparenchymal injection of allogenic placenta mesenchymal stem cells derived exosome in patients undergoing decompressive craniectomy following malignant middle cerebral artery infarct, a pilot randomized clinical trial. Int J Prev Med. 2022;13:7.
The pilot experimental study of the neuroprotective effects of exosomes in extremely low birth weight infants [Internet]. Available from: https://clinicaltrials.gov/ct2/show/NCT05490173?term=exosome&cond=brain&draw=2&rank=1.
The safety and the efficacy evaluation of allogenic adipose MSC-exos in patients with Alzheimer’s disease [Internet]. Available from: https://clinicaltrials.gov/ct2/show/NCT04388982?term=exosome&cond=brain&draw=3&rank=16.
Zhang ZW, Wei P, Zhang GJ, Yan JX, Zhang S, Liang J, et al. Intravenous infusion of the exosomes derived from human umbilical cord mesenchymal stem cells enhance neurological recovery after traumatic brain injury via suppressing the NF-kappaB pathway. Open Life Sci. 2022;17(1):189–201.
Khayatan D, Razavi SM, Arab ZN, Niknejad AH, Nouri K, Momtaz S, et al. Protective effects of curcumin against traumatic brain injury. Biomed Pharmacother. 2022;154: 113621.
Kalani A, Chaturvedi P, Kamat PK, Maldonado C, Bauer P, Joshua IG, et al. Curcumin-loaded embryonic stem cell exosomes restored neurovascular unit following ischemia-reperfusion injury. Int J Biochem Cell Biol. 2016;79:360–9.
Schmitt C, Lechanteur A, Cossais F, Bellefroid C, Arnold P, Lucius R, et al. Liposomal encapsulated curcumin effectively attenuates neuroinflammatory and reactive astrogliosis reactions in glia cells and organotypic brain slices. Int J Nanomedicine. 2020;15:3649–67.
Chao PK, Lu KT, Jhu JY, Wo YY, Huang TC, Ro LS, et al. Indomethacin protects rats from neuronal damage induced by traumatic brain injury and suppresses hippocampal IL-1beta release through the inhibition of Nogo-A expression. J Neuroinflammation. 2012;9:121.
Koulaeinejad N, Haddadi K, Ehteshami S, Shafizad M, Salehifar E, Emadian O, et al. Effects of minocycline on neurological outcomes in patients with acute traumatic brain injury: a pilot study. Iran J Pharm Res. 2019;18(2):1086–96.
Ge XT, Lei P, Wang HC, Zhang AL, Han ZL, Chen X, et al. miR-21 improves the neurological outcome after traumatic brain injury in rats. Sci Rep. 2014;4:6718.
Xu F, Liao JZ, Xiang GY, Zhao PX, Ye F, Zhao Q, et al. MiR-101 and doxorubicin codelivered by liposomes suppressing malignant properties of hepatocellular carcinoma. Cancer Med. 2017;6(3):651–61.
Yang Y, Ye Y, Su X, He J, Bai W, He X. MSCs-derived exosomes and neuroinflammation, neurogenesis and therapy of traumatic brain injury. Front Cell Neurosci. 2017;11:55.
Gao X, Chen J. Mild traumatic brain injury results in extensive neuronal degeneration in the cerebral cortex. J Neuropathol Exp Neurol. 2011;70(3):183–91.
Bagnato S, Boccagni C. Moderate/severe traumatic brain injury as a trigger of chronic neurodegeneration in humans. Neural Regen Res. 2020;15(7):1247–8.
Prevention CfDCa. Report to congress on mild traumatic brain injury in the united states: steps to prevent a serious public health problem. Atlanta, GA2003.
Author information
Authors and Affiliations
Contributions
KH and EL determined the scope of the review. KH wrote the initial draft. EL provided feedback and edits. Both contributed to the revision.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Communicated by Aliasger Salem.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hennigan, K., Lavik, E. Nature vs. Manmade: Comparing Exosomes and Liposomes for Traumatic Brain Injury. AAPS J 25, 83 (2023). https://doi.org/10.1208/s12248-023-00849-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-023-00849-8