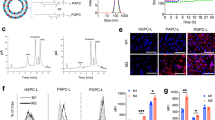
Abstract
Liposomes are frequently used in cancer therapy to encapsulate and apply anticancer drugs. Here, we show that a systemic treatment of mice bearing skin tumors with empty phosphatidylcholine liposomes (PCL) resulted in inhibition of tumor growth, which was similar to that observed with the synthetic bacterial lipoprotein and TLR1/2 agonist Pam3CSK4 (BLP). Both compounds led to a substantial decrease of macrophages in spleen and in the tumor-bearing skin. Furthermore, both treatments induced the expression of typical macrophage markers in the tumor-bearing tissue. As expected, BLP induced the expression of the M1 marker genes Cxcl10 and iNOS, whereas PCL, besides inducing iNOS, also increased the M2 marker genes Arg1 and Trem2. In vitro experiments demonstrated that neither PCL nor BLP influenced proliferation or survival of tumor cells, whereas both compounds inhibited proliferation and survival and increased the migratory capacity of bone marrow-derived macrophages (BMDM). However, in contrast to BLP, PCL did not activate cytokine secretion and induced a different BMDM phenotype. Together, the data suggest that similar to BLP, PCL induce an antitumor response by influencing the tumor microenvironment, in particular by functional alterations of macrophages, however, in a distinct manner from those induced by BLP.




Similar content being viewed by others
References
Immordino ML, Dosio F, Cattel L (2006) Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomedicine 1:297–315
Riaz M (1995) Review article: stability and uses of liposomes. Pak J Pharm Sci 8:69–79
Schwendener RA (2007) Liposomes in biology and medicine. Adv Exp Med Biol 620:117–128
Alipour M, Smith MG, Pucaj K, Suntres ZE (2012) Acute toxicity study of liposomal antioxidant formulations containing N-acetylcysteine, alpha-tocopherol, and gamma-tocopherol in rats. J Liposome Res 22(2):158–167
Papahadjopoulos D, Allen TM, Gabizon A, Mayhew E, Matthay K et al (1991) Sterically stabilized liposomes: improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc Natl Acad Sci USA 88:11460–11464
van Rooijen N, van Nieuwmegen R (1980) Liposomes in immunology: multilamellar phosphatidylcholine liposomes as a simple, biodegradable and harmless adjuvant without any immunogenic activity of its own. Immunol Commun 9:243–256
Takano S, Aramaki Y, Tsuchiya S (2003) Physicochemical properties of liposomes affecting apoptosis induced by cationic liposomes in macrophages. Pharm Res 20:962–968
Ma HM, Wu Z, Nakanishi H (2011) Phosphatidylserine-containing liposomes suppress inflammatory bone loss by ameliorating the cytokine imbalance provoked by infiltrated macrophages. Lab Invest 91:921–931
Zhang J, Fujii S, Wu Z, Hashioka S, Tanaka Y et al (2006) Involvement of COX-1 and up-regulated prostaglandin E synthases in phosphatidylserine liposome-induced prostaglandin E2 production by microglia. J Neuroimmunol 172:112–120
Graeser R, Bornmann C, Esser N, Ziroli V, Jantscheff P et al (2009) Antimetastatic effects of liposomal gemcitabine and empty liposomes in an orthotopic mouse model of pancreatic cancer. Pancreas 38:330–337
Gordon S, Taylor PR (2005) Monocyte and macrophage heterogeneity. Nat Rev Immunol 5:953–964
Lewis CE, Pollard JW (2006) Distinct role of macrophages in different tumor microenvironments. Cancer Res 66:605–612
Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A et al (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25:677–686
Mosser DM, Edwards JP (2008) Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8:958–969
Biswas SK, Mantovani A (2010) Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol 11:889–896
Ydens E, Cauwels A, Asselbergh B, Goethals S, Peeraer L et al (2012) Acute injury in the peripheral nervous system triggers an alternative macrophage response. J Neuroinflammation 9:176
Liew FY, Patel M, Xu D (2005) Toll-like receptor 2 signalling and inflammation. Ann Rheum Dis 64(Suppl 4):iv104–iv105
Zhang Y, Luo F, Cai Y, Liu N, Wang L et al (2011) TLR1/TLR2 agonist induces tumor regression by reciprocal modulation of effector and regulatory T cells. J Immunol 186:1963–1969
Zeisberger SM, Odermatt B, Marty C, Zehnder-Fjallman AH, Ballmer-Hofer K et al (2006) Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. Br J Cancer 95:272–281
Zibat A, Uhmann A, Nitzki F, Wijgerde M, Frommhold A et al (2009) Time-point and dosage of gene inactivation determine the tumor spectrum in conditional Ptch knockouts. Carcinogenesis 30:918–926
So PL, Langston AW, Daniallinia N, Hebert JL, Fujimoto MA et al (2006) Long-term establishment, characterization and manipulation of cell lines from mouse basal cell carcinoma tumors. Exp Dermatol 15:742–750
Francke A, Herold J, Weinert S, Strasser RH, Braun-Dullaeus RC (2011) Generation of mature murine monocytes from heterogeneous bone marrow and description of their properties. J Histochem Cytochem 59:813–825
Liu J, Buckley JM, Redmond HP, Wang JH (2010) ST2 negatively regulates TLR2 signaling, but is not required for bacterial lipoprotein-induced tolerance. J Immunol 184:5802–5808
Scheffel J, Regen T, Van Rossum D, Seifert S, Ribes S et al (2012) Toll-like receptor activation reveals developmental reorganization and unmasks responder subsets of microglia. Glia 60:1930–1943
Rasmussen JW, Cello J, Gil H, Forestal CA, Furie MB et al (2006) Mac-1 + cells are the predominant subset in the early hepatic lesions of mice infected with Francisella tularensis. Infect Immun 74:6590–6598
Ferron M, Vacher J (2005) Targeted expression of cre recombinase in macrophages and osteoclasts in transgenic mice. Genesis 41:138–145
D’Ambrosio D, Panina-Bordignon P, Sinigaglia F (2003) Chemokine receptors in inflammation: an overview. J Immunol Methods 273:3–13
Zhao Q (2010) Dual targeting of CCR2 and CCR5: therapeutic potential for immunologic and cardiovascular diseases. J Leukoc Biol 88:41–55
Salaun B, Coste I, Rissoan MC, Lebecque SJ, Renno T (2006) TLR3 can directly trigger apoptosis in human cancer cells. J Immunol 176:4894–4901
Geutskens SB, Nikolic T, Dardenne M, Leenen PJ, Savino W (2004) Defective up-regulation of CD49d in final maturation of NOD mouse macrophages. Eur J Imun 34:3465–3476
Aliprantis AO, Yang RB, Weiss DS, Godowski P, Zychlinsky A (2000) The apoptotic signaling pathway activated by Toll-like receptor-2. EMBO J 19:3325–3336
Luster AD (2002) The role of chemokines in linking innate and adaptive immunity. Curr Opin Immunol 14:129–135
Deshmane SL, Kremlev S, Amini S, Sawaya BE (2009) Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res 29:313–326
Murata M (2008) Activation of Toll-like receptor 2 by a novel preparation of cell wall skeleton from mycobacterium bovis BCG Tokyo (SMP-105) sufficiently enhances immune responses against tumors. Cancer Sci 99:1435–1440
Cheng N, Xia T, Han Y, He QJ, Zhao R et al (2011) Synergistic antitumor effects of liposomal honokiol combined with cisplatin in colon cancer models. Oncol Lett 2:957–962
Sakakima Y, Hayakawa A, Nagasaka T, Nakao A (2007) Prevention of hepatocarcinogenesis with phosphatidylcholine and menaquinone-4: in vitro and in vivo experiments. J Hepatol 47:83–92
Fukunaga K, Hossain Z, Takahashi K (2008) Marine phosphatidylcholine suppresses 1,2-dimethylhydrazine-induced colon carcinogenesis in rats by inducing apoptosis. Nutr Res 28:635–640
Cheng Y, Zhao Q, Liu X, Araki S, Zhang S et al (2006) Phosphatidylcholine-specific phospholipase C, p53 and ROS in the association of apoptosis and senescence in vascular endothelial cells. FEBS Lett 580:4911–4915
Li H, Lee JH, Kim SY, Yun HY, Baek KJ et al (2011) Phosphatidylcholine induces apoptosis of 3T3-L1 adipocytes. J Biomed Sci 18:91
Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS et al (2011) Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472:57–63
Harokopakis E, Hajishengallis G, Michalek SM (1998) Effectiveness of liposomes possessing surface-linked recombinant B subunit of cholera toxin as an oral antigen delivery system. Infect Immun 66:4299–4304
Richards RL, Rao M, Wassef NM, Glenn GM, Rothwell SW et al (1998) Liposomes containing lipid A serve as an adjuvant for induction of antibody and cytotoxic T-cell responses against RTS, S malaria antigen. Infect Immun 66:2859–2865
Faisal SM, Chen JW, McDonough SP, Chang CF, Teng CH et al (2011) Immunostimulatory and antigen delivery properties of liposomes made up of total polar lipids from non-pathogenic bacteria leads to efficient induction of both innate and adaptive immune responses. Vaccine 29:2381–2391
Laverman P, Dams ET, Storm G, Hafmans TG, Croes HJ et al (2001) Microscopic localization of PEG-liposomes in a rat model of focal infection. J Controlled Release 75:347–355
De Palma M, Lewis CE (2013) Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell 23:277–286
Sonoki T, Nagasaki A, Gotoh T, Takiguchi M, Takeya M et al (1997) Coinduction of nitric-oxide synthase and arginase I in cultured rat peritoneal macrophages and rat tissues in vivo by lipopolysaccharide. J Biol Chem 272:3689–3693
Turnbull IR, Gilfillan S, Cella M, Aoshi T, Miller M et al (2006) Cutting edge: TREM-2 attenuates macrophage activation. J Immunol 177:3520–3524
Gordon S, Martinez FO (2010) Alternative activation of macrophages: mechanism and functions. Immunity 32:593–604
Carr MW, Roth SJ, Luther E, Rose SS, Springer TA (1994) Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci U S A 91:3652–3656
Flaishon L, Becker-Herman S, Hart G, Levo Y, Kuziel WA et al (2004) Expression of the chemokine receptor CCR2 on immature B cells negatively regulates their cytoskeletal rearrangement and migration. Blood 104:933–941
Acknowledgments
We are grateful to Susan Peter for excellent animal care and Anke Frommhold for technical assistance. The work is supported by the DFG (FOR942 HA 2197/5-2 to Heidi Hahn).
Conflict of interest
None
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
König, S., Regen, T., Dittmann, K. et al. Empty liposomes induce antitumoral effects associated with macrophage responses distinct from those of the TLR1/2 agonist Pam3CSK4 (BLP). Cancer Immunol Immunother 62, 1587–1597 (2013). https://doi.org/10.1007/s00262-013-1444-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-013-1444-4