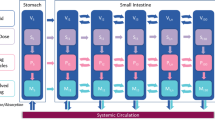
Abstract
Since the processes of dissolution and membrane permeation are affected by the water content in the gastrointestinal (GI) tract, the water dynamics in the GI tract is expected to have a significant impact on the absorption of orally administered drugs. Here, we aimed to develop a physiologically based fluid kinetic (PBFK) model using GI water kinetic parameters obtained from in situ closed-loop studies in rats in order to quantitatively predict GI water dynamics. By incorporating the experimentally measured site-specific parameters of GI water absorption and secretion into a GI compartment model, we developed a bottom-up PBFK model that successfully simulates the reported GI fluid dynamics in rats and humans observed using positron emission tomography and magnetic resonance imaging, respectively. The simulations indicate that the water volume in both the stomach and duodenum is transiently increased by water ingestion, while that in the intestine below the jejunum is unchanged and remains in a steady state in both rats and humans. Furthermore, sensitivity analysis of the effect of ingested water volume on the volume-time profiles of water in the GI tract indicated that the impact of ingested water is limited to the proximal part of the GI tract. Simulations indicated that changes in water kinetic parameters may alter the impact of the ingested water on GI fluid dynamics, especially in the proximal part. Incorporating this PBFK model into a physiologically based pharmacokinetic (PBPK) absorption model has the potential to predict oral drug absorption in a variety of GI water environments.
Graphical Abstract









Similar content being viewed by others
Abbreviations
- AIC :
-
Akaike’s information criterion
- ATOM:
-
Advanced translocation model
- AUC:
-
Area under the fluid volume-time curve
- AUMC:
-
Area under the first-moment curve
- BCS:
-
Biopharmaceutics Classification System
- DFCAT:
-
Dynamic fluid compartment absorption and transit
- GI:
-
Gastrointestinal
- k abs :
-
Absorption rate constant of water
- k sec :
-
Secretion rate constant of water
- k transit :
-
Transit rate constant
- MRI:
-
Magnetic resonance imaging
- MRT:
-
Mean residence time
- PBFK:
-
Physiologically based fluid kinetic(s)
- PBPK:
-
Physiologically based pharmacokinetic(s)
- PET:
-
Positron emission tomography
- Q por :
-
Portal vein blood flow
- Q villi :
-
Villous blood flow
- S :
-
Absorptive surface area
- S sum :
-
The sum of the absorptive surface area of the GI tract
- SF abs :
-
Scaling factor for water absorption in humans
- SF abs_rat :
-
In situ-in vivo Scaling factor for water absorption in rats
- SF sec :
-
Scaling factor for water secretion in humans
- v :
-
Velocity
- V :
-
Volume
- v abs :
-
Water absorption rate
- V max :
-
Maximum volume
- v sec :
-
Water secretion rate
- V ss :
-
Steady-state volume
- v transit :
-
Transit rate
- WSS :
-
Weighted sum of squares
References
Masaoka Y, Tanaka Y, Kataoka M, Sakuma S, Yamashita S. Site of drug absorption after oral administration: assessment of membrane permeability and luminal concentration of drugs in each segment of gastrointestinal tract. Eur J Pharm Sci. 2006;29(3–4):240–50.
Suzuki S, Shirasaka Y, Okada R, Eguchi A, Kishimoto H, Langguth P, et al. Quantitative analysis of the effect of controlled-release formulation on nonlinear gastrointestinal absorption of P-glycoprotein substrate talinolol using physiologically based pharmacokinetic absorption model. J Drug Deliv Sci Technol. 2020;56:101057.
Ichijo K, Oda R, Ishihara M, Okada R, Moteki Y, Funai Y, et al. Osmolality of orally administered solutions influences luminal water volume and drug absorption in intestine. J Pharm Sci. 2017;106(9):2889–94.
Funai Y, Shirasaka Y, Ishihara M, Takemura M, Ichijo K, Kishimoto H, et al. Effect of osmolality on the pharmacokinetic interaction between apple juice and atenolol in rats. Drug Metab Dispos. 2019;47(4):386–91.
Jeon H, Jang IJ, Lee S, Ohashi K, Kotegawa T, Ieiri I, et al. Apple juice greatly reduces systemic exposure to atenolol. Br J Clin Pharmacol. 2013;75(1):172–9.
Takemura M, Tanaka Y, Inoue K, Tamai I, Shirasaka Y. Influence of osmolality on gastrointestinal fluid volume and drug absorption: potential impact on oral salt supplementation. J Pharm Health Care Sci. 2021;7(1):29.
Funai Y, Takemura M, Inoue K, Shirasaka Y. Effect of ingested fluid volume and solution osmolality on intestinal drug absorption: impact on drug interaction with beverages. Eur J Pharm Sci. 2022;172:106136.
Hintz RJ, Johnson KC. The effect of particle size distribution on dissolution rate and oral absorption. Int J Pharm. 1989;51:9–17.
Hisaka A, Sugiyama Y. Analysis of nonlinear and nonsteady state hepatic extraction with the dispersion model using the finite difference method. J Pharmacokinet Biopharm. 1998;26(5):495–519.
Takashima T, Shingaki T, Katayama Y, Hayashinaka E, Wada Y, Kataoka M, et al. Dynamic analysis of fluid distribution in the gastrointestinal tract in rats: positron emission tomography imaging after oral administration of nonabsorbable marker, [(18)F]Deoxyfluoropoly(ethylene glycol). Mol Pharm. 2013;10(6):2261–9.
Haruta S, Kawai K, Jinnouchi S, Ogawara KI, Higaki K, Tamura S, et al. Evaluation of absorption kinetics of orally administered theophylline in rats based on gastrointestinal transit monitoring by gamma scintigraphy. J Pharm Sci. 2001;90(4):464–73.
Takahashi H, Nishikawa M, Hayashi M, Awazu S. The use of a perfluorochemical emulsion as a vascular perfusate in drug absorption. J Pharm Pharmacol. 1988;40:252–7.
Matsuda Y, Konno Y, Hashimoto T, Nagai M, Taguchi T, Satsukawa M, et al. Quantitative assessment of intestinal first-pass metabolism of oral drugs using portal-vein cannulated rats. Pharm Res. 2015;32(2):604–16.
Sasaki Y, Wagner HN Jr. Measurement of the distribution of cardiac output in unanesthetized rats. J Appl Physiol. 1971;30(6):879–84.
Mudie DM, Murray K, Hoad CL, Pritchard SE, Garnett MC, Amidon GL, et al. Quantification of gastrointestinal liquid volumes and distribution following a 240 mL dose of water in the fasted state. Mol Pharm. 2014;11(9):3039–47.
Murray K, Hoad CL, Mudie DM, Wright J, Heissam K, Abrehart N, et al. Magnetic resonance imaging quantification of fasted state colonic liquid pockets in healthy humans. Mol Pharm. 2017;14(8):2629–38.
Yamashita S, Takashima T, Kataoka M, Oh H, Sakuma S, Takahashi M, et al. PET imaging of the gastrointestinal absorption of orally administered drugs in conscious and anesthetized rats. J Nucl Med. 2011;52(2):249–56.
Yu A, Jackson T, Tsume Y, Koenigsknecht M, Wysocki J, Marciani L, et al. Mechanistic fluid transport model to estimate gastrointestinal fluid volume and its dynamic change over time. AAPS J. 2017;19(6):1682–90.
Asano S, Yoshitomo A, Hozuki S, Sato H, Kazuki Y, Hisaka A. A new intestinal model for analysis of drug absorption and interactions considering physiological translocation of contents. Drug Metab Dispos. 2021;49(7):581–91.
Yu A, Koenigsknecht MJ, Hens B, Baker JR, Wen B, Jackson TL, et al. Mechanistic deconvolution of oral absorption model with dynamic gastrointestinal fluid to predict regional rate and extent of GI drug dissolution. AAPS J. 2019;22(1):3.
Tanaka Y, Higashino H, Kataoka M, Yamashita S. In vivo fluid volume in rat gastrointestinal tract: kinetic analysis on the luminal concentration of nonabsorbable FITC-dextran after oral administration. J Pharm Sci. 2020;109(6):1863–6.
Kambayashi A, Shirasaka Y. Food effects on gastrointestinal physiology and drug absorption. Drug Metab Pharmacokinet. 2023;48:10048.
Shay H, Sun DCH. Etiology and pathology of gastric and duodenal ulcer. In: Bockus HL, editors. Gastroenterology, 2nd ed, Vol 1. Philadelphia & London: WB Saunders; 1963. p. 420.
Kulnigg-Dabsch S. Autoimmune gastritis. Wien Med Wochenschr. 2016;166(13–14):424–30.
Woosley RL, Echt DS, Roden DM. Effects of congestive heart failure on the pharmacokinetics and pharmacodynamics of antiarrhythmic agents. Am J Cardiol. 1986;57(3):25B-33B.
Ogawa R, Stachnik JM, Echizen H. Clinical pharmacokinetics of drugs in patients with heart failure: an update (part 1, drugs administered intravenously). Clin Pharmacokinet. 2013;52(3):169–85.
Ouzounian JG, Elkayam U. Physiologic changes during normal pregnancy and delivery. Cardiol Clin. 2012;30(3):317–29.
Jamei M, Turner D, Yang J, Neuhoff S, Polak S, Rostami-Hodjegan A, Tucker G. Population-based mechanistic prediction of oral drug absorption. AAPS J. 2009;11(2):225–37.
Funding
This work was supported in part by a Grant-in-Aid for JSPS Fellows [JP21J12535] and a Grant-in-Aid for Scientific Research (C) [JP20K07165] from the Japan Society for the Promotion of Science (JSPS), a Research Grant from the Mochida Memorial Foundation for Medical and Pharmaceutical Research, a grant from the Research Foundation for Pharmaceutical Sciences, and a grant from Nakatomi Foundation.
Author information
Authors and Affiliations
Contributions
Participated in research design: Suzuki, Shirasaka.
Conducted experiments: Suzuki, Shirasaka.
Performed data analysis: Suzuki, Shirasaka.
Wrote or contributed to the writing of the manuscript: Suzuki, Inoue, Tamai, Shirasaka.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Suzuki, S., Inoue, K., Tamai, I. et al. Quantitative Analysis of Gastrointestinal Water Dynamics by Means of a Physiologically Based Fluid Kinetic Model. AAPS J 25, 42 (2023). https://doi.org/10.1208/s12248-023-00809-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-023-00809-2