Abstract
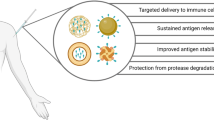
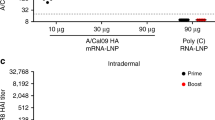
Influenza is a global health concern with millions of infections occurring yearly. Seasonal flu vaccines are one way to combat this virus; however, they are poorly protective against influenza as the virus is constantly mutating, particularly at the immunodominant hemagglutinin (HA) head group. A more broadly acting approach involves Computationally Optimized Broadly Reactive Antigen (COBRA). COBRA HA generates a broad immune response that is capable of protecting against mutating strains. Unfortunately, protein-based vaccines are often weekly immunogenic, so to help boost the immune response, we employed the use of acetalated dextran (Ace-DEX) microparticles (MPs) two ways: one to conjugate COBRA HA to the surface and a second to encapsulate cGAMP. To conjugate the COBRA HA to the surface of the Ace-DEX MPs, a poly(L-lactide)-polyethylene glycol co-polymer with a vinyl sulfone terminal group (PLLA-PEG-VS) was used. MPs encapsulating the STING agonist cGAMP were co-delivered with the antigen to form a broadly active influenza vaccine. This vaccine approach was evaluated in vivo with a prime-boost-boost vaccination schedule and illustrated generation of a humoral and cellular response that could protect against a lethal challenge of A/California/07/2009 in BALB/c mice.
Graphical Abstract





Similar content being viewed by others
References
Potter CW. A history of influenza. J Appl Microbiol. 2001;91(4):572–9. https://doi.org/10.1046/j.1365-2672.2001.01492.x.
Akin L, Gozel MG. Understanding dynamics of pandemics. Turk J Med Sci. 2020;50(SI-1):515–9. https://doi.org/10.3906/sag-2004-133.
Wilks S, de Graaf M, Smith DJ, Burke DF. A review of influenza haemagglutinin receptor binding as it relates to pandemic properties. Vaccine. 2012;30(29):4369–76. https://doi.org/10.1016/j.vaccine.2012.02.076.
Kim H, Webster RG, Webby RJ. Influenza virus: dealing with a drifting and shifting pathogen. Viral Immunol. 2018;31(2):174–83. https://doi.org/10.1089/vim.2017.0141.
Allen JD, Ray S, Ross TM. Split inactivated cobra vaccine elicits protective antibodies against h1n1 and h3n2 influenza viruses. PLoS ONE. 2018;13(9):e0204284. https://doi.org/10.1371/journal.pone.0204284.
Allen JD, Ross TM. Bivalent H1 and H3 cobra recombinant hemagglutinin vaccines elicit seroprotective antibodies against H1n1 and H3n2 influenza viruses from 2009 to 2019. J Virol. 2022;96(7):e0165221. https://doi.org/10.1128/jvi.01652-21.
Carter DM, Darby CA, Lefoley BC, Crevar CJ, Alefantis T, Oomen R, et al. Design and characterization of a computationally optimized broadly reactive hemagglutinin vaccine for H1n1 influenza viruses. J Virol. 2016;90(9):4720–34. https://doi.org/10.1128/jvi.03152-15.
Crevar CJ, Carter DM, Lee KY, Ross TM. Cocktail of H5n1 cobra Ha vaccines elicit protective antibodies against H5n1 viruses from multiple clades. Hum Vaccin Immunother. 2015;11(3):572–83. https://doi.org/10.1080/21645515.2015.1012013.
Darricarrère N, Pougatcheva S, Duan X, Rudicell RS, Chou TH, DiNapoli J, et al. Development of a Pan-H1 influenza vaccine. J Virol. 2018;92(22). https://doi.org/10.1128/jvi.01349-18.
Giles BM, Bissel SJ, Dealmeida DR, Wiley CA, Ross TM. Antibody breadth and protective efficacy are increased by vaccination with computationally optimized hemagglutinin but not with polyvalent hemagglutinin-based H5n1 virus-like particle vaccines. Clin Vaccine Immunol. 2012;19(2):128–39. https://doi.org/10.1128/CVI.05533-11.
Giles BM, Crevar CJ, Carter DM, Bissel SJ, Schultz-Cherry S, Wiley CA, et al. A computationally optimized hemagglutinin virus-like particle vaccine elicits broadly reactive antibodies that protect nonhuman primates from H5n1 infection. J Infect Dis. 2012;205(10):1562–70. https://doi.org/10.1093/infdis/jis232.
Sautto GA, Kirchenbaum GA, Abreu RB, Ecker JW, Pierce SR, Kleanthous H, et al. A computationally optimized broadly reactive antigen subtype-specific influenza vaccine strategy elicits unique potent broadly neutralizing antibodies against hemagglutinin. J Immunol. 2020;204(2):375–85. https://doi.org/10.4049/jimmunol.1900379.
Varma DM, Batty CJ, Stiepel RT, Graham-Gurysh EG, Roque JA 3rd, Pena ES, et al. Development of an intranasal gel for the delivery of a broadly acting subunit influenza vaccine. ACS Biomater Sci Eng. 2022;8(4):1573–82. https://doi.org/10.1021/acsbiomaterials.2c00015.
Wong TM, Allen JD, Bebin-Blackwell AG, Carter DM, Alefantis T, DiNapoli J, et al. Computationally optimized broadly reactive hemagglutinin elicits hemagglutination inhibition antibodies against a panel of H3n2 influenza virus cocirculating variants. J Virol. 2017;91(24). https://doi.org/10.1128/JVI.01581-17.
Pulendran B, P SA, O'Hagan DT. Emerging concepts in the science of vaccine adjuvants. Nat Rev Drug Discov. 2021;20(6):454–75. https://doi.org/10.1038/s41573-021-00163-y.
Genito CJ, Batty CJ, Bachelder EM, Ainslie KM. Considerations for size, surface charge, polymer degradation, co-delivery, and manufacturability in the development of polymeric particle vaccines for infectious diseases. Adv Nanobiomed Res. 2021;1(3):2000041. https://doi.org/10.1002/anbr.202000041.
Beaudette TT, Bachelder EM, Cohen JA, Obermeyer AC, Broaders KE, Frechet JM, et al. In vivo studies on the effect of co-encapsulation of Cpg DNA and antigen in acid-degradable microparticle vaccines. Mol Pharm. 2009;6(4):1160–9. https://doi.org/10.1021/mp900038e.
Allahyari M, Mohit E. Peptide/protein vaccine delivery system based on Plga particles. Hum Vaccin Immunother. 2016;12(3):806–28. https://doi.org/10.1080/21645515.2015.1102804.
Gu P, Wusiman A, Wang S, Zhang Y, Liu Z, Hu Y, et al. Polyethylenimine-coated Plga nanoparticles-encapsulated angelica sinensis polysaccharide as an adjuvant to enhance immune responses. Carbohydr Polym. 2019;223:115128. https://doi.org/10.1016/j.carbpol.2019.115128.
Bachelder EM, Beaudette TT, Broaders KE, Dashe J, Frechet JM. Acetal-derivatized dextran: an acid-responsive biodegradable material for therapeutic applications. J Am Chem Soc. 2008;130(32):10494–5. https://doi.org/10.1021/ja803947s.
Kauffman KJ, Do C, Sharma S, Gallovic MD, Bachelder EM, Ainslie KM. Synthesis and characterization of acetalated dextran polymer and microparticles with ethanol as a degradation product. ACS Appl Mater Interfaces. 2012;4(8):4149–55. https://doi.org/10.1021/am3008888.
Broaders KE, Cohen JA, Beaudette TT, Bachelder EM, Frechet JM. Acetalated dextran is a chemically and biologically tunable material for particulate immunotherapy. Proc Natl Acad Sci U S A. 2009;106(14):5497–502. https://doi.org/10.1073/pnas.0901592106.
Chen N, Collier MA, Gallovic MD, Collins GC, Sanchez CC, Fernandes EQ, et al. Degradation of acetalated dextran can be broadly tuned based on cyclic acetal coverage and molecular weight. Int J Pharm. 2016;512(1):147–57. https://doi.org/10.1016/j.ijpharm.2016.08.031.
Junkins RD, Gallovic MD, Johnson BM, Collier MA, Watkins-Schulz R, Cheng N, et al. A robust microparticle platform for a sting-targeted adjuvant that enhances both humoral and cellular immunity during vaccination. J Control Release. 2018;270:1–13. https://doi.org/10.1016/j.jconrel.2017.11.030.
Gallovic MD, Schully KL, Bell MG, Elberson MA, Palmer JR, Darko CA, et al. Acetalated dextran microparticulate vaccine formulated via coaxial electrospray preserves toxin neutralization and enhances murine survival following inhalational Bacillus anthracis exposure. Adv Healthc Mater. 2016;5(20):2617–27. https://doi.org/10.1002/adhm.201600642.
Chen N, Johnson MM, Collier MA, Gallovic MD, Bachelder EM, Ainslie KM. Tunable degradation of acetalated dextran microparticles enables controlled vaccine adjuvant and antigen delivery to modulate adaptive immune responses. J Control Release. 2018;273:147–59. https://doi.org/10.1016/j.jconrel.2018.01.027.
Singh A, Eliciting B. Cell Immunity against infectious diseases using nanovaccines. Nat Nanotechnol. 2021;16(1):16–24. https://doi.org/10.1038/s41565-020-00790-3.
Kato Y, Abbott RK, Freeman BL, Haupt S, Groschel B, Silva M, et al. Multifaceted effects of antigen valency on B cell response composition and differentiation in vivo. Immunity. 2020;53(3):548-63.e8. https://doi.org/10.1016/j.immuni.2020.08.001.
Pejawar-Gaddy S, Kovacs JM, Barouch DH, Chen B, Irvine DJ. Design of lipid nanocapsule delivery vehicles for multivalent display of recombinant Env trimers in Hiv vaccination. Bioconjug Chem. 2014;25(8):1470–8. https://doi.org/10.1021/bc5002246.
Gil-Ocana V, Jimenez IM, Mayorga C, Dona I, Cespedes JA, Montanez MI, et al. Multiepitope dendrimeric antigen-silica particle composites as nano-based platforms for specific recognition of Iges. Front Immunol. 2021;12:750109. https://doi.org/10.3389/fimmu.2021.750109.
Morales-Sanfrutos J, Lopez-Jaramillo J, Ortega-Munoz M, Megia-Fernandez A, Perez-Balderas F, Hernandez-Mateo F, et al. Vinyl sulfone: a versatile function for simple bioconjugation and immobilization. Org Biomol Chem. 2010;8(3):667–75. https://doi.org/10.1039/b920576d.
Chen N, Collier MA, Gallovic MD, Collins GC, Sanchez CC, Fernandes EQ, et al. Degradation of acetalated dextran can be broadly tuned based on cyclic acetal coverage and molecular weight. Int J Pharm. 2016;512(1):147–57. https://doi.org/10.1016/j.ijpharm.2016.08.031.
Chen N, Gallovic MD, Tiet P, Ting JP, Ainslie KM, Bachelder EM. Investigation of tunable acetalated dextran microparticle platform to optimize M2e-based influenza vaccine efficacy. J Control Release. 2018;289:114–24. https://doi.org/10.1016/j.jconrel.2018.09.020.
Huang Y, Franca MS, Allen JD, Shi H, Ross TM. Next generation of computationally optimized broadly reactive Ha vaccines elicited cross-reactive immune responses and provided protection against H1n1 virus infection. Vaccines (Basel). 2021;9(7). https://doi.org/10.3390/vaccines9070793.
Ecker JW, Kirchenbaum GA, Pierce SR, Skarlupka AL, Abreu RB, Cooper RE, et al. High-yield expression and purification of recombinant influenza virus proteins from stably-transfected mammalian cell lines. Vaccines (Basel). 2020;8(3). https://doi.org/10.3390/vaccines8030462.
Watkins-Schulz R, Tiet P, Gallovic MD, Junkins RD, Batty C, Bachelder EM, et al. A microparticle platform for sting-targeted immunotherapy enhances natural killer cell- and Cd8(+) T cell-mediated anti-tumor immunity. Biomaterials. 2019;205:94–105. https://doi.org/10.1016/j.biomaterials.2019.03.011.
Eckshtain-Levi M, Batty CJ, Lifshits LM, McCammitt B, Moore KM, Amouzougan EA, et al. Metal-organic coordination polymer for delivery of a subunit broadly acting influenza vaccine. ACS Appl Mater Interfaces. 2022;14(25):28548–58. https://doi.org/10.1021/acsami.2c04671.
Earnest JT, Basore K, Roy V, Bailey AL, Wang D, Alter G, et al. Neutralizing antibodies against Mayaro virus require Fc effector functions for protective activity. J Exp Med. 2019;216(10):2282–301. https://doi.org/10.1084/jem.20190736.
Butler AL, Fallon JK, Alter G. A sample-sparing multiplexed Adcp assay. Front Immunol. 2019;10:1851. https://doi.org/10.3389/fimmu.2019.01851.
Boudreau CM, Yu WH, Suscovich TJ, Talbot HK, Edwards KM, Alter G. Selective induction of antibody effector functional responses using Mf59-adjuvanted vaccination. J Clin Invest. 2020;130(2):662–72. https://doi.org/10.1172/JCI129520.
Zelek WM, Harris CL, Morgan BP. Extracting the barbs from complement assays: identification and optimisation of a safe substitute for traditional buffers. Immunobiology. 2018;223(12):744–9. https://doi.org/10.1016/j.imbio.2018.07.016.
Pati R, Shevtsov M, Sonawane A. Nanoparticle vaccines against infectious diseases. Front Immunol. 2018;9:2224. https://doi.org/10.3389/fimmu.2018.02224.
Batty CJ, Gallovic MD, Williams J, Ross TM, Bachelder EM, Ainslie KM. Multiplexed electrospray enables high throughput production of cGAMP microparticles to serve as an adjuvant for a broadly acting influenza vaccine. Int J Pharm. 2022;622:121839. https://doi.org/10.1016/j.ijpharm.2022.121839.
Gallovic MD, Junkins RD, Sandor AM, Pena ES, Sample CJ, Mason AK, et al. Sting agonist-containing microparticles improve seasonal influenza vaccine efficacy and durability in ferrets over standard adjuvant. J Control Release. 2022;347:356–68. https://doi.org/10.1016/j.jconrel.2022.05.017.
Gallovic MD, Schully KL, Bell MG, Elberson MA, Palmer JR, Darko CA, et al. Acetalated dextran microparticulate vaccine formulated via coaxial electrospray preserves toxin neutralization and enhances murine survival following inhalational Bacillus anthracis exposure. Adv Healthc Mater. 2016. https://doi.org/10.1002/adhm.201600642.
Ko EJ, Kang SM. Immunology and efficacy of Mf59-adjuvanted vaccines. Hum Vaccin Immunother. 2018;14(12):3041–5. https://doi.org/10.1080/21645515.2018.1495301.
Miyauchi K, Sugimoto-Ishige A, Harada Y, Adachi Y, Usami Y, Kaji T, et al. Protective neutralizing influenza antibody response in the absence of T follicular helper cells. Nat Immunol. 2016;17(12):1447–58. https://doi.org/10.1038/ni.3563.
Huber VC, McKeon RM, Brackin MN, Miller LA, Keating R, Brown SA, et al. Distinct contributions of vaccine-induced immunoglobulin G1 (Igg1) and Igg2a antibodies to protective immunity against influenza. Clin Vaccine Immunol. 2006;13(9):981–90. https://doi.org/10.1128/CVI.00156-06.
Lu LL, Suscovich TJ, Fortune SM, Alter G. Beyond binding: antibody effector functions in infectious diseases. Nat Rev Immunol. 2018;18(1):46–61. https://doi.org/10.1038/nri.2017.106.
Vanderven HA, Kent SJ. The protective potential of Fc-mediated antibody functions against influenza virus and other viral pathogens. Immunol Cell Biol. 2020;98(4):253–63. https://doi.org/10.1111/imcb.12312.
Vafa O, Gilliland GL, Brezski RJ, Strake B, Wilkinson T, Lacy ER, et al. An engineered Fc variant of an igg eliminates all immune effector functions Via structural perturbations. Methods. 2014;65(1):114–26. https://doi.org/10.1016/j.ymeth.2013.06.035.
Lee NK, Wang CJ, Lim J, Park W, Kwon HK, Kim SN, et al. Impact of the conjugation of antibodies to the surfaces of polymer nanoparticles on the immune cell targeting abilities. Nano Converg. 2021;8(1):24. https://doi.org/10.1186/s40580-021-00274-7.
Wang Y, van Asdonk K, Zijlstra P. A robust and general approach to quantitatively conjugate enzymes to plasmonic nanoparticles. Langmuir. 2019;35(41):13356–63. https://doi.org/10.1021/acs.langmuir.9b01879.
McCarthy DP, Hunter ZN, Chackerian B, Shea LD, Miller SD. Targeted immunomodulation using antigen-conjugated nanoparticles. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2014;6(3):298–315. https://doi.org/10.1002/wnan.1263.
Alam F, Singh A, Flores-Malavet V, Sell S, Cooper AM, Swain SL, et al. Cd25-Targeted Il-2 signals promote improved outcomes of influenza infection and boost memory Cd4 T cell formation. J Immunol. 2020;204(12):3307–14. https://doi.org/10.4049/jimmunol.2000205.
Bot A, Bot S, Bona CA. Protective role of gamma interferon during the recall response to influenza virus. J Virol. 1998;72(8):6637–45. https://doi.org/10.1128/JVI.72.8.6637-6645.1998.
Hanson MC, Bershteyn A, Crespo MP, Irvine DJ. Antigen delivery by lipid-enveloped PLGA microparticle vaccines mediated by in situ vesicle shedding. Biomacromol. 2014;15(7):2475–81. https://doi.org/10.1021/bm500337r.
Sia ZR, He X, Zhang A, Ang JC, Shao S, Seffouh A, et al. A liposome-displayed hemagglutinin vaccine platform protects mice and ferrets from heterologous influenza virus challenge. Proc Natl Acad Sci U S A. 2021;118(22). https://doi.org/10.1073/pnas.2025759118.
Acknowledgements
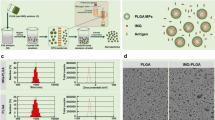
Abstract figure and Fig. 1b were in part created with BioRender.com. This work was performed in part at the Chapel Hill Analytical and Nanofabrication Laboratory, CHANL, a member of the North Carolina Research Triangle Nanotechnology Network, RTNN, which is supported by the National Science Foundation, Grant ECCS-2025064, as part of the National Nanotechnology Coordinated Infrastructure, NNCI. We thank Dr. Amar S. Kumbhar of CHANL for his assistance with SEM characterization.
Funding
Funding for this work was supported by National Institutes of Health (NIH) NIAID Collaborative Influenza Vaccine Innovation Centers (CIVICs) Contract #75N93019C00052 (PI: Ross) and NIH R01AI147497 (PI: Ainslie). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
Kristy M. Ainslie (principal investigator) and Eric M. Bachelder (co-principal investigator) designed the experiments and wrote the first draft of manuscript. Cole J. Batty and Liubov M. Lifshits contributed to writing of manuscript, and other co-authors provided minor edits. Cole J. Batty and Liubov M. Lifshits prepared figures for the manuscript. Ted M. Ross (co-principal investigator) provided COBRA HA for the experiments. Michael A. Carlock conducted HAI experiment. Liubov M. Lifshits, Dylan H. Hendy, and Meital Eckshtain-Levi carried out synthesis and characterization of materials. Cole J. Batty, Dylan A. Hendy, and Luis Ontiveros-Padilla carried out in vivo experiments. Dylan H. Hendy revised the manuscript in response to reviewer’s comments.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Batty, C.J., Lifshits, L.M., Hendy, D.A. et al. Vinyl Sulfone-functionalized Acetalated Dextran Microparticles as a Subunit Broadly Acting Influenza Vaccine. AAPS J 25, 22 (2023). https://doi.org/10.1208/s12248-023-00786-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-023-00786-6