ABSTRACT
Inhibitory effects of asunaprevir, daclatasvir, grazoprevir, paritaprevir, simeprevir, and voxilaprevir, direct-acting antiviral (DAA) drugs for the treatment of chronic hepatitis C virus (HCV) infection, were evaluated in vitro against a range of clinically important drug transporters. In vitro inhibition studies were conducted using transporter transfected cells and membrane vesicles. The risk of clinical drug-drug interactions (DDIs) was assessed using simplified static models recommended by regulatory agencies. Furthermore, we refined and developed static models to predict complex DDIs with several statins (pitavastatin, rosuvastatin, atorvastatin, and pravastatin) by mechanistically assessing differential inhibitory effects of perpetrator drugs on multiple transporters, such as organic anion transporting polypeptides (OATP1B), breast cancer resistance protein (BCRP), multidrug resistance protein 2 (MRP2), organic anion transporter 3 (OAT3), and cytochrome P450 CYP3A enzyme, as they are known to contribute to absorption, distribution, metabolism and excretion (ADME) of above statins. These models successfully predicted a total of 46 statin DDIs, including above DAA drugs and their fix-dose combination regimens. Predicted plasma area under curve ratio (AUCR) with and without perpetrator drugs was within ~ 2-fold of observed values. In contrast, simplified static R-value model resulted in increased false negative and false positive predictions when different prediction cut-off values were applied. Our studies suggest that mechanistic static model is a promising and useful tool to provide more accurate prediction of the risk and magnitude of DDIs with statins in early drug development and may help to improve the management of clinical DDIs for HCV drugs to ensure effective and safe HCV therapy.

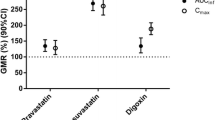
GRAPHICAL ABSTRACT


Similar content being viewed by others
Abbreviations
- ADME:
-
Absorption, distribution, metabolism, and excretion
- AUCR:
-
Area under the curve ratio
- AAFE:
-
Absolute average fold error
- BCRP:
-
Breast cancer resistance protein
- BSP:
-
Bromosulfophthalein
- CYP:
-
Cytochrome P450
- Cmax :
-
Maximum plasma concentration
- DAA:
-
Direct-acting antiviral
- DDI:
-
Drug-drug interaction
- DME:
-
Drug metabolizing enzymes
- ES:
-
Estrone sulfate
- EMA:
-
European Medical Agency
- FDA:
-
U.S. Food and Drug Administration
- ft :
-
Fraction transported
- Fa :
-
Fraction absorbed
- Fg :
-
The fraction escaping intestinal metabolism
- HCV:
-
Hepatitis C virus
- Iin,max :
-
Maximal inhibition concentration at the liver inlet
- I1u :
-
Mean steady state unbound Cmax
- I2 :
-
Dose of the inhibitor (in mol)/250 mL
- MDR:
-
Multidrug resistance
- MTX:
-
Methotrexate
- OATP:
-
Organic anion transporting polypeptide
- OAT:
-
Organic anion transporter
- OCT:
-
Organic cation transporter
- P-gp:
-
P-glycoprotein
- PK:
-
Pharmacokinetics
- RMSE:
-
Root mean squared error
References
Neant N, Solas C. Drug-drug interactions potential of direct-acting antivirals for the treatment of chronic hepatitis C virus infection. Int J Antimicrob Agents. 2020;56(1):105571. https://doi.org/10.1016/j.ijantimicag.2018.10.014.
Garrison KL, German P, Mogalian E, Mathias A. The drug-drug interaction potential of antiviral agents for the treatment of chronic hepatitis C infection. Drug Metab Dispos. 2018;46(8):1212–25. https://doi.org/10.1124/dmd.117.079038.
Hill L. Hepatitis C virus direct-acting antiviral drug interactions and use in renal and hepatic impairment. Top Antivir Med. 2015;23(2):92–6.
Talavera Pons S, Boyer A, Lamblin G, Chennell P, Chatenet FT, Nicolas C, et al. Managing drug-drug interactions with new direct-acting antiviral agents in chronic hepatitis C. Br J Clin Pharmacol. 2017;83(2):269–93. https://doi.org/10.1111/bcp.13095.
Soriano V, Labarga P, Barreiro P, Fernandez-Montero JV, de Mendoza C, Esposito I, Benítez-Gutiérrez L, Peña JM. Drug interactions with new hepatitis C oral drugs. Expert Opin Drug Metab Toxicol. 2015;11(3):333–41. https://doi.org/10.1517/17425255.2015.998997.
Kiser JJ, Burton JR Jr, Everson GT. Drug-drug interactions during antiviral therapy for chronic hepatitis C. Nat Rev Gastroenterol Hepatol. 2013;10(10):596–606. https://doi.org/10.1038/nrgastro.2013.106.
Asante-Appiah E, Curry S, McMonagle P, Ingravallo P, Chase R, Nickle D, et al. Antiviral activity and resistance analysis of NS3/4A protease inhibitor grazoprevir and NS5A inhibitor elbasvir in hepatitis C virus GT4 replicons. Antimicrob Agents Chemother. 2017;61(7). https://doi.org/10.1128/AAC.00363-17.
Shebley M, Liu J, Kavetskaia O, Sydor J, de Morais SM, Fischer V, Nijsen MJMA, Bow DAJ. Mechanisms and predictions of drug-drug interactions of the hepatitis C virus three direct-acting antiviral regimen: paritaprevir/ritonavir, ombitasvir, and dasabuvir. Drug Metab Dispos. 2017;45(7):755–64. https://doi.org/10.1124/dmd.116.074518.
Eley T, Garimella T, Li W, Bertz RJ. Asunaprevir: a review of preclinical and clinical pharmacokinetics and drug-drug interactions. Clin Pharmacokinet. 2015;54(12):1205–22. https://doi.org/10.1007/s40262-015-0299-6.
Eley T, Han YH, Huang SP, He B, Li W, Bedford W, Stonier M, Gardiner D, Sims K, Rodrigues AD, Bertz RJ. Organic anion transporting polypeptide-mediated transport of, and inhibition by, asunaprevir, an inhibitor of hepatitis C virus NS3 protease. Clin Pharmacol Ther. 2015;97(2):159–66. https://doi.org/10.1002/cpt.4.
Garimella T, You X, Wang R, Huang SP, Kandoussi H, Bifano M, Bertz R, Eley T. A review of daclatasvir drug-drug interactions. Adv Ther. 2016;33(11):1867–84. https://doi.org/10.1007/s12325-016-0407-5.
Ouwerkerk-Mahadevan S, Snoeys J, Peeters M, Beumont-Mauviel M, Simion A. Drug-drug interactions with the NS3/4A protease inhibitor simeprevir. Clin Pharmacokinet. 2016;55(2):197–208. https://doi.org/10.1007/s40262-015-0314-y.
Yoshida K, Maeda K, Sugiyama Y. Hepatic and intestinal drug transporters: prediction of pharmacokinetic effects caused by drug-drug interactions and genetic polymorphisms. Annu Rev Pharmacol Toxicol. 2013;53:581–612. https://doi.org/10.1146/annurev-pharmtox-011112-140309.
Shitara Y, Maeda K, Ikejiri K, Yoshida K, Horie T, Sugiyama Y. Clinical significance of organic anion transporting polypeptides (OATPs) in drug disposition: their roles in hepatic clearance and intestinal absorption. Biopharm Drug Dispos. 2013;34(1):45–78. https://doi.org/10.1002/bdd.1823.
Chu X, Liao M, Shen H, Yoshida K, Zur AA, Arya V, Galetin A, Giacomini KM, Hanna I, Kusuhara H, Lai Y, Rodrigues D, Sugiyama Y, Zamek-Gliszczynski MJ, Zhang L, on behalf of the International Transporter Consortium. Clinical probes and endogenous biomarkers as substrates for transporter drug-drug interaction evaluation: perspectives from the international transporter consortium. Clin Pharmacol Ther. 2018;104(5):836–64. https://doi.org/10.1002/cpt.1216.
Camerino GM, Tarantino N, Canfora I, De Bellis M, Musumeci O, Pierno S. Statin-Induced myopathy: translational studies from preclinical to clinical evidence. Int J Mol Sci. 2021;22(4). https://doi.org/10.3390/ijms22042070.
FDA C. In Vitro Drug Interaction Studies — Cytochrome P450 Enzyme- and Transporter-Mediated Drug Interactions Guidance for Industry. 2020 [updated January 2020; cited https://www.fda.gov/media/134582/download].
EMA C. Guideline on the investigation of drug interactions European Medicines Agency. https://wwwemaeuropaeu/documents/scientific-guideline/guideline-investigation-drug-interactions_enpdf. 2012;(Committee for Human Medicinal Products (CHMP)).
Elsby R, Hilgendorf C, Fenner K. Understanding the critical disposition pathways of statins to assess drug-drug interaction risk during drug development: it’s not just about OATP1B1. Clin Pharmacol Ther. 2012;92(5):584–98. https://doi.org/10.1038/clpt.2012.163.
Yoshikado T, Yoshida K, Kotani N, Nakada T, Asaumi R, Toshimoto K, Maeda K, Kusuhara H, Sugiyama Y. Quantitative analyses of hepatic OATP-mediated interactions between statins and inhibitors using PBPK modeling with a parameter optimization method. Clin Pharmacol Ther. 2016;100(5):513–23. https://doi.org/10.1002/cpt.391.
Jamei M, Bajot F, Neuhoff S, Barter Z, Yang J, Rostami-Hodjegan A, Rowland-Yeo K. A mechanistic framework for in vitro-in vivo extrapolation of liver membrane transporters: prediction of drug-drug interaction between rosuvastatin and cyclosporine. Clin Pharmacokinet. 2014;53(1):73–87. https://doi.org/10.1007/s40262-013-0097-y.
Duan P, Zhao P, Zhang L. Physiologically based pharmacokinetic (PBPK) modeling of pitavastatin and atorvastatin to predict drug-drug interactions (DDIs). Eur J Drug Metab Pharmacokinet. 2017;42(4):689–705. https://doi.org/10.1007/s13318-016-0383-9.
Varma MV, Lai Y, Feng B, Litchfield J, Goosen TC, Bergman A. Physiologically based modeling of pravastatin transporter-mediated hepatobiliary disposition and drug-drug interactions. Pharm Res. 2012;29(10):2860–73. https://doi.org/10.1007/s11095-012-0792-7.
Reig-Lopez J, Garcia-Arieta A, Mangas-Sanjuan V, Merino-Sanjuan M. Current evidence, challenges, and opportunities of physiologically based pharmacokinetic models of atorvastatin for decision making. Pharmaceutics. 2021;13(5). https://doi.org/10.3390/pharmaceutics13050709.
Morse BL, Alberts JJ, Posada MM, Rehmel J, Kolur A, Tham LS, Loghin C, Hillgren KM, Hall SD, Dickinson GL. Physiologically-based pharmacokinetic modeling of atorvastatin incorporating delayed gastric emptying and acid-to-lactone conversion. CPT Pharmacometrics Syst Pharmacol. 2019;8(9):664–75. https://doi.org/10.1002/psp4.12447.
Bowman CM, Ma F, Mao J, Chen Y. Examination of physiologically-based pharmacokinetic models of rosuvastatin. CPT Pharmacometrics Syst Pharmacol. 2021;10(1):5–17. https://doi.org/10.1002/psp4.12571.
Taskar KS, Pilla Reddy V, Burt H, Posada MM, Varma M, Zheng M, Ullah M, Emami Riedmaier A, Umehara KI, Snoeys J, Nakakariya M, Chu X, Beneton M, Chen Y, Huth F, Narayanan R, Mukherjee D, Dixit V, Sugiyama Y, Neuhoff S. Physiologically-based pharmacokinetic models for evaluating membrane transporter mediated drug-drug interactions: current capabilities, case studies, future opportunities, and Recommendations. Clin Pharmacol Ther. 2020;107(5):1082–115. https://doi.org/10.1002/cpt.1693.
Zamek-Gliszczynski MJ, Kalvass JC, Pollack GM, Brouwer KL. Relationship between drug/metabolite exposure and impairment of excretory transport function. Drug Metab Dispos. 2009;37(2):386–90. https://doi.org/10.1124/dmd.108.023648.
Tod M, Bourguignon L, Bleyzac N, Goutelle S. Quantitative prediction of interactions mediated by transporters and cytochromes: application to organic anion transporting polypeptides, breast cancer resistance protein and cytochrome 2C8. Clin Pharmacokinet. 2020;59(6):757–70. https://doi.org/10.1007/s40262-019-00853-2.
Varma MV, Bi YA, Kimoto E, Lin J. Quantitative prediction of transporter- and enzyme-mediated clinical drug-drug interactions of organic anion-transporting polypeptide 1B1 substrates using a mechanistic net-effect model. J Pharmacol Exp Ther. 2014;351(1):214–23. https://doi.org/10.1124/jpet.114.215970.
Sane R, Cheung KWK, Kovacs P, Farasyn T, Li R, Bui A, et al. Calibrating the in vitro-in vivo correlation for OATP mediated drug-drug interactions with rosuvastatin using static and PBPK models. Drug Metab Dispos. 2020;48:1264–70. https://doi.org/10.1124/dmd.120.000149.
Chan G, Houle R, Lin M, Yabut J, Cox K, Wu J, Chu X. Role of transporters in the disposition of a novel beta-lactamase inhibitor: relebactam (MK-7655). J Antimicrob Chemother. 2019;74(7):1894–903. https://doi.org/10.1093/jac/dkz101.
Prueksaritanont T, Tatosian DA, Chu X, Railkar R, Evers R, Chavez-Eng C, Lutz R, Zeng W, Yabut J, Chan GH, Cai X, Latham AH, Hehman J, Stypinski D, Brejda J, Zhou C, Thornton B, Bateman KP, Fraser I, Stoch SA. Validation of a microdose probe drug cocktail for clinical drug interaction assessments for drug transporters and CYP3A. Clin Pharmacol Ther. 2017;101(4):519–30. https://doi.org/10.1002/cpt.525.
Chu X, Cai X, Cui D, Tang C, Ghosal A, Chan G, Green MD, Kuo Y, Liang Y, Maciolek CM, Palamanda J, Evers R, Prueksaritanont T. In vitro assessment of drug-drug interaction potential of boceprevir associated with drug metabolizing enzymes and transporters. Drug Metab Dispos. 2013;41(3):668–81. https://doi.org/10.1124/dmd.112.049668.
Rizk ML, Houle R, Chan GH, Hafey M, Rhee EG, Chu X. Raltegravir has a low propensity to cause clinical drug interactions through inhibition of major drug transporters: an in vitro evaluation. Antimicrob Agents Chemother. 2014;58(3):1294–301. https://doi.org/10.1128/AAC.02049-13.
Boerekamps A, De Weggheleire A, van den Berk GE, Lauw FN, Claassen MAA, Posthouwer D, et al. Treatment of acute hepatitis C genotypes 1 and 4 with 8 weeks of grazoprevir plus elbasvir (DAHHS2): an open-label, multicentre, single-arm, phase 3b trial. Lancet Gastroenterol Hepatol. 2019;4(4):269–77. https://doi.org/10.1016/S2468-1253(18)30414-X.
Cook JA, Feng B, Fenner KS, Kempshall S, Liu R, Rotter C, Smith DA, Troutman MD, Ullah M, Lee CA. Refining the in vitro and in vivo critical parameters for P-glycoprotein, [I]/IC50 and [I2]/IC50, that allow for the exclusion of drug candidates from clinical digoxin interaction studies. Mol Pharm. 2010;7(2):398–411. https://doi.org/10.1021/mp900174z.
Balimane PV, Marino A, Chong S. P-gp inhibition potential in cell-based models: which “calculation” method is the most accurate? AAPS J. 2008;10(4):577–86. https://doi.org/10.1208/s12248-008-9068-x.
Vaidyanathan J, Yoshida K, Arya V, Zhang L. Comparing various in vitro prediction criteria to assess the potential of a new molecular entity to inhibit organic anion transporting polypeptide 1B1. J Clin Pharmacol. 2016;56(Suppl 7):S59–72. https://doi.org/10.1002/jcph.723.
Yoshida K, Zhao P, Zhang L, Abernethy DR, Rekic D, Reynolds KS, et al. In vitro-in vivo extrapolation of metabolism- and transporter-mediated drug-drug interactions-overview of basic prediction methods. J Pharm Sci. 2017;106(9):2209–13. https://doi.org/10.1016/j.xphs.2017.04.045.
Bi YA, Scialis RJ, Lazzaro S, Mathialagan S, Kimoto E, Keefer J, Zhang H, Vildhede AM, Costales C, Rodrigues AD, Tremaine LM, Varma MVS. Reliable rate measurements for active and passive hepatic uptake using plated human hepatocytes. AAPS J. 2017;19(3):787–96. https://doi.org/10.1208/s12248-017-0051-2.
Yoshida K, Maeda K, Sugiyama Y. Transporter-mediated drug--drug interactions involving OATP substrates: predictions based on in vitro inhibition studies. Clin Pharmacol Ther. 2012;91(6):1053–64. https://doi.org/10.1038/clpt.2011.351.
Prueksaritanont T, Chu X, Evers R, Klopfer SO, Caro L, Kothare PA, Dempsey C, Rasmussen S, Houle R, Chan G, Cai X, Valesky R, Fraser IP, Stoch SA. Pitavastatin is a more sensitive and selective organic anion-transporting polypeptide 1B clinical probe than rosuvastatin. Br J Clin Pharmacol. 2014;78(3):587–98. https://doi.org/10.1111/bcp.12377.
Kunze A, Huwyler J, Camenisch G, Poller B. Prediction of organic anion-transporting polypeptide 1B1- and 1B3-mediated hepatic uptake of statins based on transporter protein expression and activity data. Drug Metab Dispos. 2014;42(9):1514–21. https://doi.org/10.1124/dmd.114.058412.
Hinton LK, Galetin A, Houston JB. Multiple inhibition mechanisms and prediction of drug-drug interactions: status of metabolism and transporter models as exemplified by gemfibrozil-drug interactions. Pharm Res. 2008;25(5):1063–74. https://doi.org/10.1007/s11095-007-9446-6.
Martin PD, Warwick MJ, Dane AL, Brindley C, Short T. Absolute oral bioavailability of rosuvastatin in healthy white adult male volunteers. Clin Ther. 2003;25(10):2553–63. https://doi.org/10.1016/s0149-2918(03)80316-8.
Keskitalo JE, Zolk O, Fromm MF, Kurkinen KJ, Neuvonen PJ, Niemi M. ABCG2 polymorphism markedly affects the pharmacokinetics of atorvastatin and rosuvastatin. Clin Pharmacol Ther. 2009;86(2):197–203. https://doi.org/10.1038/clpt.2009.79.
Bi YA, Costales C, Mathialagan S, West M, Eatemadpour S, Lazzaro S, Tylaska L, Scialis R, Zhang H, Umland J, Kimoto E, Tess DA, Feng B, Tremaine LM, Varma MVS, Rodrigues AD. Quantitative contribution of six major transporters to the hepatic uptake of drugs: “SLC-phenotyping” using primary human hepatocytes. J Pharmacol Exp Ther. 2019;370(1):72–83. https://doi.org/10.1124/jpet.119.257600.
Feng B, Varma MV. Evaluation and quantitative prediction of renal transporter-mediated drug-drug interactions. J Clin Pharmacol. 2016;56(Suppl 7):S110–21. https://doi.org/10.1002/jcph.702.
Ando H, Tsuruoka S, Yanagihara H, Sugimoto K, Miyata M, Yamazoe Y, Takamura T, Kaneko S, Fujimura A. Effects of grapefruit juice on the pharmacokinetics of pitavastatin and atorvastatin. Br J Clin Pharmacol. 2005;60(5):494–7. https://doi.org/10.1111/j.1365-2125.2005.02462.x.
Fukazawa I, Uchida N, Uchida E, Yasuhara H. Effects of grapefruit juice on pharmacokinetics of atorvastatin and pravastatin in Japanese. Br J Clin Pharmacol. 2004;57(4):448–55. https://doi.org/10.1046/j.1365-2125.2003.02030.x.
Vildhede A, Karlgren M, Svedberg EK, Wisniewski JR, Lai Y, Noren A, et al. Hepatic uptake of atorvastatin: influence of variability in transporter expression on uptake clearance and drug-drug interactions. Drug Metab Dispos. 2014;42(7):1210–8. https://doi.org/10.1124/dmd.113.056309.
Maeda K, Ikeda Y, Fujita T, Yoshida K, Azuma Y, Haruyama Y, Yamane N, Kumagai Y, Sugiyama Y. Identification of the rate-determining process in the hepatic clearance of atorvastatin in a clinical cassette microdosing study. Clin Pharmacol Ther. 2011;90(4):575–81. https://doi.org/10.1038/clpt.2011.142.
Lau YY, Huang Y, Frassetto L, Benet LZ. effect of OATP1B transporter inhibition on the pharmacokinetics of atorvastatin in healthy volunteers. Clin Pharmacol Ther. 2007;81(2):194–204. https://doi.org/10.1038/sj.clpt.6100038.
Fahmi OA, Hurst S, Plowchalk D, Cook J, Guo F, Youdim K, Dickins M, Phipps A, Darekar A, Hyland R, Obach RS. Comparison of different algorithms for predicting clinical drug-drug interactions, based on the use of CYP3A4 in vitro data: predictions of compounds as precipitants of interaction. Drug Metab Dispos. 2009;37(8):1658–66. https://doi.org/10.1124/dmd.108.026252.
Obach RS, Walsky RL, Venkatakrishnan K. Mechanism-based inactivation of human cytochrome p450 enzymes and the prediction of drug-drug interactions. Drug Metab Dispos. 2007;35(2):246–55. https://doi.org/10.1124/dmd.106.012633.
Singhvi SM, Pan HY, Morrison RA, Willard DA. Disposition of pravastatin sodium, a tissue-selective HMG-CoA reductase inhibitor, in healthy subjects. Br J Clin Pharmacol. 1990;29(2):239–43. https://doi.org/10.1111/j.1365-2125.1990.tb03626.x.
Everett DW, Chando TJ, Didonato GC, Singhvi SM, Pan HY, Weinstein SH. Biotransformation of pravastatin sodium in humans. Drug Metab Dispos. 1991;19(4):740–8.
Itagaki S, Chiba M, Kobayashi M, Hirano T, Iseki K. Contribution of multidrug resistance-associated protein 2 to secretory intestinal transport of organic anions. Biol Pharm Bull. 2008;31(1):146–8. https://doi.org/10.1248/bpb.31.146.
Garimella T, Tao X, Sims K, Chang YT, Rana J, Myers E, Wind-Rotolo M, Bhatnagar R, Eley T, LaCreta F, AbuTarif M. Effects of a fixed-dose co-formulation of daclatasvir, asunaprevir, and beclabuvir on the pharmacokinetics of a cocktail of cytochrome P450 and drug transporter substrates in healthy subjects. Drugs R D. 2018;18(1):55–65. https://doi.org/10.1007/s40268-017-0222-8.
Furihata T, Matsumoto S, Fu Z, Tsubota A, Sun Y, Matsumoto S, Kobayashi K, Chiba K. Different interaction profiles of direct-acting anti-hepatitis C virus agents with human organic anion transporting polypeptides. Antimicrob Agents Chemother. 2014;58(8):4555–64. https://doi.org/10.1128/AAC.02724-14.
Kunze A, Ediage EN, Dillen L, Monshouwer M, Snoeys J. Clinical investigation of coproporphyrins as sensitive biomarkers to predict mild to strong OATP1B-mediated drug-drug interactions. Clin Pharmacokinet. 2018;57(12):1559–70. https://doi.org/10.1007/s40262-018-0648-3.
Shitara Y, Sugiyama Y. Preincubation-dependent and long-lasting inhibition of organic anion transporting polypeptide (OATP) and its impact on drug-drug interactions. Pharmacol Ther. 2017;177:67–80. https://doi.org/10.1016/j.pharmthera.2017.02.042.
Tatrai P, Schweigler P, Poller B, Domange N, de Wilde R, Hanna I, et al. A systematic in vitro investigation of the inhibitor preincubation effect on multiple classes of clinically relevant transporters. Drug Metab Dispos. 2019;47(7):768–78. https://doi.org/10.1124/dmd.118.085993.
Amundsen R, Christensen H, Zabihyan B, Asberg A. Cyclosporine A, but not tacrolimus, shows relevant inhibition of organic anion-transporting protein 1B1-mediated transport of atorvastatin. Drug Metab Dispos. 2010;38(9):1499–504. https://doi.org/10.1124/dmd.110.032268.
Shitara Y, Takeuchi K, Nagamatsu Y, Wada S, Sugiyama Y, Horie T. Long-lasting inhibitory effects of cyclosporin A, but not tacrolimus, on OATP1B1- and OATP1B3-mediated uptake. Drug Metab Pharmacokinet. 2012;27(4):368–78. https://doi.org/10.2133/dmpk.dmpk-11-rg-096.
Shitara Y, Takeuchi K, Horie T. Long-lasting inhibitory effects of saquinavir and ritonavir on OATP1B1-mediated uptake. J Pharm Sci. 2013;102(9):3427–35. https://doi.org/10.1002/jps.23477.
Caro L, Prueksaritanont T, Fandozzi CM, Feng HP, Guo Z, Wolford D, Panebianco D, Fraser IP, Levine V, Swearingen D, Butterton JR, Iwamoto M, Yeh WW. Evaluation of pharmacokinetic drug interactions of the direct-acting antiviral agents elbasvir and grazoprevir with pitavastatin, rosuvastatin, pravastatin, and atorvastatin in healthy adults. Clin Drug Investig. 2021;41(2):133–47. https://doi.org/10.1007/s40261-020-00974-8.
Chu X, Galetin A, Zamek-Gliszczynski MJ, Zhang L, Tweedie DJ, International Transporter C. Dabigatran etexilate and digoxin: comparison as clinical probe substrates for evaluation of P-gp inhibition. Clin Pharmacol Ther. 2018;104(5):788–92. https://doi.org/10.1002/cpt.1213.
Bifano M, Hwang C, Oosterhuis B, Hartstra J, Grasela D, Tiessen R, Velinova-Donga M, Kandoussi H, Sevinsky H, Bertz R. Assessment of pharmacokinetic interactions of the HCV NS5A replication complex inhibitor daclatasvir with antiretroviral agents: ritonavir-boosted atazanavir, efavirenz and tenofovir. Antivir Ther. 2013;18(7):931–40. https://doi.org/10.3851/IMP2674.
Watanabe T, Kusuhara H, Maeda K, Kanamaru H, Saito Y, Hu Z, Sugiyama Y. Investigation of the rate-determining process in the hepatic elimination of HMG-CoA reductase inhibitors in rats and humans. Drug Metab Dispos. 2010;38(2):215–22. https://doi.org/10.1124/dmd.109.030254.
Pfeifer ND, Yang K, Brouwer KL. Hepatic basolateral efflux contributes significantly to rosuvastatin disposition I: characterization of basolateral versus biliary clearance using a novel protocol in sandwich-cultured hepatocytes. J Pharmacol Exp Ther. 2013;347(3):727–36. https://doi.org/10.1124/jpet.113.207472.
Johnson M, Patel D, Matheny C, Ho M, Chen L, Ellens H. Inhibition of intestinal OATP2B1 by the calcium receptor antagonist ronacaleret results in a significant drug-drug interaction by causing a 2-fold decrease in exposure of rosuvastatin. Drug Metab Dispos. 2017;45(1):27–34. https://doi.org/10.1124/dmd.116.072397.
Zamek-Gliszczynski MJ, Taub ME, Chothe PP, Chu X, Giacomini KM, Kim RB, Ray AS, Stocker SL, Unadkat JD, Wittwer MB, Xia C, Yee SW, Zhang L, Zhang Y, International Transporter Consortium. Transporters in drug development: 2018 ITC recommendations for transporters of emerging clinical importance. Clin Pharmacol Ther. 2018;104(5):890–9. https://doi.org/10.1002/cpt.1112.
Kinzi J, Grube M, Meyer Zu Schwabedissen HE. OATP2B1 - The underrated member of the organic anion transporting polypeptide family of drug transporters? Biochem Pharmacol. 2021;188:114534. https://doi.org/10.1016/j.bcp.2021.114534.
Yu J, Zhou Z, Tay-Sontheimer J, Levy RH, Ragueneau-Majlessi I. Intestinal drug interactions mediated by OATPs: a systematic review of preclinical and clinical findings. J Pharm Sci. 2017;106(9):2312–25. https://doi.org/10.1016/j.xphs.2017.04.004.
McFeely SJ, Wu L, Ritchie TK, Unadkat J. Organic anion transporting polypeptide 2B1 - More than a glass-full of drug interactions. Pharmacol Ther. 2019;196:204–15. https://doi.org/10.1016/j.pharmthera.2018.12.009.
Keskitalo JE, Kurkinen KJ, Neuvoneni PJ, Niemi M. ABCB1 haplotypes differentially affect the pharmacokinetics of the acid and lactone forms of simvastatin and atorvastatin. Clin Pharmacol Ther. 2008;84(4):457–61. https://doi.org/10.1038/clpt.2008.25.
Deng F, Tuomi SK, Neuvonen M, Hirvensalo P, Kulju S, Wenzel C, Oswald S, Filppula AM, Niemi M. Comparative Hepatic and Intestinal Efflux Transport of Statins. Drug Metab Dispos. 2021;49(9):750–9. https://doi.org/10.1124/dmd.121.000430.
Prueksaritanont T, Subramanian R, Fang X, Ma B, Qiu Y, Lin JH, Pearson PG, Baillie TA. Glucuronidation of statins in animals and humans: a novel mechanism of statin lactonization. Drug Metab Dispos. 2002;30(5):505–12. https://doi.org/10.1124/dmd.30.5.505.
Tsamandouras N, Dickinson G, Guo Y, Hall S, Rostami-Hodjegan A, Galetin A, Aarons L. Development and application of a mechanistic pharmacokinetic model for simvastatin and its active metabolite simvastatin acid using an integrated population PBPK approach. Pharm Res. 2015;32(6):1864–83. https://doi.org/10.1007/s11095-014-1581-2.
Acknowledgements
We acknowledge Dr. Thomayant Prueksaritanont for valuable suggestions on study design. We also acknowledge Ms. Xiaoxin Cai and Dr. Jingjing Guo for technical assistance, and Drs. Lucinda Hittle and Alema Galijatovic-Idrizbegovic for critical review of the manuscript.
Funding
All studies conducted in this manuscript were sponsored by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Author information
Authors and Affiliations
Contributions
Participated in research design: Chu, Chan, Houle, Fandozzi
Conducted experiments: Chan, Houle, Lin
Contributed new reagents or analytic tools: NA
Performed data analysis: Chu, Chan, Houle, Lin, Yabut
Wrote or contributed to the writing of the manuscript: Chu, Chan, Houle, Yabut, Fandozzi
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations
Rights and permissions
About this article
Cite this article
Chu, X., Chan, G.H., Houle, R. et al. In Vitro Assessment of Transporter Mediated Perpetrator DDIs for Several Hepatitis C Virus Direct-Acting Antiviral Drugs and Prediction of DDIs with Statins Using Static Models. AAPS J 24, 45 (2022). https://doi.org/10.1208/s12248-021-00677-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-021-00677-8