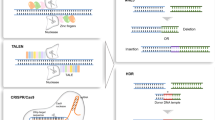
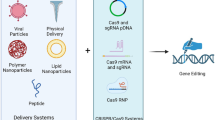
Abstract
Improvements in the understanding of human genetics and its roles in disease development and prevention have led to an increased interest in therapeutic genome editing via the use of engineered nucleases. Various approaches have been explored in the past focusing on the development of an effective and safe system for sequence-specific editing. Compared to earlier nucleases such as zinc finger nuclease and transcription activator-like effector nuclease, the relatively low cost and ease of producing clustered regularly interspaced short palindromic repeats associated protein 9 (CRISPR/Cas9) systems have made therapeutic genome editing significantly more feasible. CRISPR/Cas9 genome editing has shown great potential to correct genetic mutations implicated in monogenic diseases and to eradicate latent or chronic viral infections in preclinical studies. Several CRISPR/Cas9-based therapeutics have reached the clinical stage, including treatments for inherited red blood cell disorders and Leber Congenital Amaurosis 10, as well as CRISPR/Cas9-edited T cells designed to target and destroy cancer cells. Further advances in therapeutic genome editing will rely on a safe and more efficient method of in vivo CRISPR/Cas9 delivery and improved efficiency of homology-directed repair for site-specific gene insertion or replacement. While other reviews have focused on one or two aspects of CRISPR/Cas9 genome editing, this review aims to provide a summary of the mechanisms of genome editing, the reasons for the emerging interest in CRISPR/Cas9 compared to other engineered nucleases, the current progress in developing CRISPR/Cas9 delivery systems, and the current preclinical and clinical applications of CRISPR/Cas9 genome editing.



Similar content being viewed by others
References
Willyard C. New human gene tally reignites debate. Nature. 2018;558:354–5.
Prakash V, Moore M, Yáñez-Muñoz R. Current progress in therapeutic gene editing for monogenic diseases. Mol Ther. 2016;24:465–74.
Chang H, Pannunzio N, Adachi N, Lieber M. Non-homologous DNA end joining and alternative pathways to double-strand break repair. Nat Rev Mol Cell Biol. 2017;18:495–506.
Liu M, Rehman S, Tang X, Gu K, Fan Q, Chen D, et al. Methodologies for improving HDR efficiency. Front Genet. 2019;9. https://doi.org/10.3389/fgene.2018.00691.
Cassandri M, Smirnov A, Novelli F, Pitolli C, Agostini M, Malewicz M et al. Zinc-finger proteins in health and disease. Cell Death Discov. 2017;3(1). https://doi.org/10.1038/cddiscovery.2017.71.
Gupta R, Musunuru K. Expanding the genetic editing tool kit: ZFNs, TALENs, and CRISPR-Cas9. J Clin Invest. 2014;124:4154–61.
Ramirez C, Foley J, Wright D, Müller-Lerch F, Rahman S, Cornu T, et al. Unexpected failure rates for modular assembly of engineered zinc fingers. Nat Methods. 2008;5:374–5.
Moore R, Chandrahas A, Bleris L. Transcription activator-like effectors: a toolkit for synthetic biology. ACS Synth Biol. 2014;3:708–16.
Nishimasu H, Ran F, Hsu P, Konermann S, Shehata S, Dohmae N, et al. Crystal structure of Cas9 in complex with guide RNA and target DNA. Cell. 2014;156:935–49.
Rath D, Amlinger L, Rath A, Lundgren M. The CRISPR-Cas immune system: biology, mechanisms and applications. Biochimie. 2015;117:119–28.
Cameron P, Coons M, Klompe S, Lied A, Smith S, Vidal B, et al. Harnessing type I CRISPR–Cas systems for genome engineering in human cells. Nat Biotechnol. 2019;37:1471–7.
McMahon S, Zhu W, Graham S, Rambo R, White M, Gloster T. Structure and mechanism of a type III CRISPR defense DNA nuclease activated by cyclic oligoadenylate. Nat Commun. 2020;11(1). https://doi.org/10.1038/s41467-019-14222-x.
Paul B, Montoya G. CRISPR-Cas12a: functional overview and applications. Biom J. 2020;43(1):8–17.
O’Connell M. Molecular mechanisms of RNA targeting by Cas13-containing type VI CRISPR–Cas systems. J Mol Biol. 2019;431:66–87.
Naeem M, Majeed S, Hoque M, Ahmad I. Latest developed strategies to minimize the off-target effects in CRISPR-Cas-mediated genome editing. Cells. 2020;9:1608. https://doi.org/10.3390/cells9071608.
Gough V, Gersbach C. Immunity to Cas9 as an obstacle to persistent genome editing. Mol Ther. 2020;28:1389–91.
Liu C, Zhang L, Liu H, Cheng K. Delivery strategies of the CRISPR-Cas9 gene-editing system for therapeutic applications. J Control Release. 2017;266:17–26.
Xu X, Wan T, Xin H, Li D, Pan H, Wu J et al. Delivery of CRISPR/Cas9 for therapeutic genome editing. J Gene Med. 2019;21(7). https://doi.org/10.1002/jgm.3107
Modzelewski A, Chen S, Willis B, Lloyd K, Wood J, He L. Efficient mouse genome engineering by CRISPR-EZ technology. Nat Protoc. 2018;13:1253–74.
Alghadban S, Bouchareb A, Hinch R, Hernandez-Pliego P, Biggs D, Preece C, et al. Electroporation and genetic supply of Cas9 increase the generation efficiency of CRISPR/Cas9 knock-in alleles in C57BL/6J mouse zygotes. Sci Rep-UK. 2020;10(1). https://doi.org/10.1038/s41598-020-74960-7.
Nüssing S, House I, Kearney C, Chen A, Vervoort S, Beavis P, et al. Efficient CRISPR/Cas9 gene editing in uncultured naive mouse T cells for in vivo studies. J Immunol. 2020;204:2308–15.
Yu J. Electroporation of CRISPR-Cas9 into malignant B cells for loss-of-function studies of target gene via knockout. Methods Mol Biol. 2020;2050:85–90.
Dean D. Microinjection. In Brenner’s Encyclopedia of Genetics (2nd etition), Maloy S. Hughe K. edit., Academic Press, 2013; pp409-10.
Neumann E, Schaefer-Ridder M, Wang Y, Hofschneider P. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1982;1:841–5.
Lissandrello C, Santos J, Hsi P, Welch M, Mott V, Kim E, et al. High-throughput continuous-flow microfluidic electroporation of mRNA into primary human T cells for applications in cellular therapy manufacturing. Sci Rep-UK. 2020;10(1). https://doi.org/10.1038/s41598-020-73755-0.
Xu C, Ruan M, Mahajan V, Tsang S. Viral delivery systems for CRISPR. Viruses. 2019;11(1):28. https://doi.org/10.3390/v11010028.
Chung S, Mollhoff I, Nguyen U, Nguyen A, Stucka N, Tieu E, et al. Factors impacting efficacy of AAV-mediated CRISPR-based genome editing for treatment of choroidal neovascularization. Mol Ther Methods Clin. 2020;17:409–17.
Muruve D. The innate immune response to adenovirus vectors. Hum Gene Ther. 2004;15:1157–66.
Ricobaraza A, Gonzalez-Aparicio M, Mora-Jimenez L, Lumbreras S, Hernandez-Alcoceba R. High-capacity adenoviral vectors: expanding the scope of gene therapy. Int J Mol Sci. 2020;21(10):3643. https://doi.org/10.3390/ijms21103643.
Schiwon M, Ehrke-Schulz E, Oswald A, Bergmann T, Michler T, Protzer U, et al. One-vector system for multiplexed CRISPR/Cas9 against hepatitis B virus cccDNA utilizing high-capacity adenoviral vectors. Mol Ther-Nucl Acids. 2018;12:242–53.
Ehrke-Schulz E, Heinemann S, Schulte L, Schiwon M, Ehrhardt A. Adenoviral vectors armed with papillomavirus oncogene specific CRISPR/Cas9 kill human-papillomavirus-induced cervical cancer cells. Cancers. 2020;12(7):1934. https://doi.org/10.3390/cancers12071934.
White M, Whittaker R, Gándara C, Stoll E. A guide to approaching regulatory considerations for lentiviral-mediated gene therapies. Hum Gene Ther Method. 2017;28:163–76.
Joglekar A, Sandoval S. Pseudotyped lentiviral vectors: one vector, many guises. Hum Gene Ther Method. 2017;28:291–301.
Milone M, O’Doherty U. Clinical use of lentiviral vectors. Leukemia. 2018;32:1529–41.
Aregger M, Chandrashekhar M, Tong A, Chan K, Moffat J. Pooled lentiviral CRISPR-Cas9 screens for functional genomics in mammalian cells. Methods Mol Biol. 2018; pp169-88.
Ortinski P, O’Donovan B, Dong X, Kantor B. Integrase-deficient lentiviral vector as an all-in-one platform for highly efficient CRISPR/Cas9-mediated gene editing. Mol Ther Methods Clin. 2017;5:153–64.
Daya S, Berns K. Gene therapy using adeno-associated virus vectors. Clin Microbiol Rev. 2008;21:583–93.
Srivastava A. In vivo tissue-tropism of adeno-associated viral vectors. Curr Opin Virol. 2016;21:75–80.
Mitani K, Kubo S. Adenovirus as an integrating vector. Curr Gene Ther. 2002;2:135–44.
Follenzi A, Santambrogio L, Annoni A. Immune responses to lentiviral vectors. Curr Gene Ther. 2007;7(5):306–15.
Tong S, Moyo B, Lee C, Leong K, Bao G. Engineered materials for in vivo delivery of genome-editing machinery. Nature Reviews Materials. 2019;4(11):726–37.
Li L, Hu S, Chen X. Non-viral delivery systems for CRISPR/Cas9-based genome editing: Challenges and opportunities. Biomaterials. 2018;171:207–18. https://doi.org/10.1016/j.biomaterials.2018.04.031.
Cui S, Wang Y, Gong Y, Lin X, Zhao Y, Zhi D, et al. Correlation of the cytotoxic effects of cationic lipids with their headgroups. Toxicol Res-UK. 2018;7:473–9.
Givens B, Naguib Y, Geary S, Devor E, Salem A. Nanoparticle-based delivery of CRISPR/Cas9 genome-editing therapeutics. AAPS J. 2018;20(6). https://doi.org/10.1208/s12248-018-0267-9.
Guo A, Wang Y, Xu S, Zhang X, Li M, Liu Q, et al. Preparation and evaluation of pH-responsive charge-convertible ternary complex FA-PEI-CCA/PEI/DNA with low cytotoxicity and efficient gene delivery. Colloid Surface B. 2017;152:58–67.
Strojan K, Lojk J, Bregar V, Veranič P, Pavlin M. Glutathione reduces cytotoxicity of polyethyleneimine coated magnetic nanoparticles in CHO cells. Toxicol in Vitro. 2017;41:12–20.
Huang Q, Li S, Ding Y, Yin H, Wang L, Wang R. Macrocycle-wrapped polyethylenimine for gene delivery with reduced cytotoxicity. Biomater Sci-UK. 2018;6:1031–9. https://doi.org/10.1039/C8BM00022K.
Chou S, Yang P, Ban Q, Yang Y, Wang M, Chien C, et al. Dual supramolecular nanoparticle vectors enable CRISPR/Cas9-mediated knockin of retinoschisin 1 gene: a potential nonviral therapeutic solution for X-linked juvenile retinoschisis. Adv Sci. 2020;7(10):1903432.
Venault A, Huang Y, Lo J, Chou C, Chinnathambi A, Higuchi A, et al. Tunable PEGylation of branch-type PEI/DNA polyplexes with a compromise of low cytotoxicity and high transgene expression: in vitro and in vivo gene delivery. J Mater Chem B. 2017;5:4732–44.
Fan Y, Sahdev P, Ochyl LJ, Akerberg J, Moon J. Cationic liposome–hyaluronic acid hybrid nanoparticles for intranasal vaccination with subunit antigens. J Control Release. 2015;208:121–9.
Qian Y, Liang X, Yang J, Zhao C, Nie W, Liu L, et al. Hyaluronan reduces cationic liposome-induced toxicity and enhances the antitumor effect of targeted gene delivery in mice. ACS Appl Mater Interfaces. 2018;10:32006–16.
Liu Y, Cao Z, Xu C, Lu Z, Luo Y, Wang J. Optimization of lipid-assisted nanoparticle for disturbing neutrophils-related inflammation. Biomaterials. 2018;172:92–104.
Luo Y, Xu C, Li H, Cao Z, Liu J, Wang J, et al. Macrophage-specific in vivo gene editing using cationic lipid-assisted polymeric nanoparticles. ACS Nano. 2018;12(2):994–1005.
Luo Y, Liang L, Gan Y, Liu J, Zhang Y, Fan Y, et al. An all-in-one nanomedicine consisting of CRISPR-Cas9 and an autoantigen peptide for restoring specific immune tolerance. ACS Appl Mater Interfaces. 2020;12:48259–71.
Alsaiari S, Patil S, Alyami M, Alamoudi K, Aleisa F, Merzaban J, et al. Endosomal escape and delivery of CRISPR/Cas9 genome editing machinery enabled by nanoscale zeolitic imidazolate framework. J Am Chem Soc. 2017;140:143–6.
Wang Y, Shahi P, Xie R, Zhang H, Abdeen A, Yodsanit N, et al. A pH-responsive silica–metal–organic framework hybrid nanoparticle for the delivery of hydrophilic drugs, nucleic acids, and CRISPR-Cas9 genome-editing machineries. J Control Release. 2020;324:194–203.
Zhang B, Wu P, Zou J, Jiang J, Zhao R, Luo B, et al. Efficient CRISPR/Cas9 gene-chemo synergistic cancer therapy via a stimuli-responsive chitosan-based nanocomplex elicits anti-tumorigenic pathway effect. Chem Eng J. 2020;393:124688. https://doi.org/10.1016/j.cej.2020.124688.
Gao X, Jin Z, Tan X, Zhang C, Zou C, Zhang W, et al. Hyperbranched poly(β-amino ester) based polyplex nanoparticles for delivery of CRISPR/Cas9 system and treatment of HPV infection associated cervical cancer. J Control Release. 2020;321:654–68.
Zhao X, Glass Z, Chen J, Yang L, Kaplan D, Xu Q. mRNA delivery using bioreducible lipidoid nanoparticles facilitates neural differentiation of human mesenchymal stem cells. Adv Healthc Mater. 2020;2000938:2000938. https://doi.org/10.1002/adhm.202000938.
Lee K, Conboy M, Park H, Jiang F, Kim H, Dewitt M, et al. Nanoparticle delivery of Cas9 ribonucleoprotein and donor DNA in vivo induces homology-directed DNA repair. Nat Biomed Eng. 2017;1:889–901.
Ray M, Lee Y, Hardie J, Mout R, Yeşilbag Tonga G, Farkas M, et al. CRISPRed macrophages for cell-based cancer immunotherapy. Bioconjug Chem. 2018;29:445–50.
Wang P, Zhang L, Xie Y, Wang N, Tang R, Zheng W, et al. Genome editing for cancer therapy: delivery of Cas9 protein/sgRNA plasmid via a gold nanocluster/lipid core-shell nanocarrier. Adv Sci. 2017;4(11):1700175. https://doi.org/10.1002/advs.201700175.
Zhang L, Wang L, Xie Y, Wang P, Deng S, Qin A, et al. Triple-targeting delivery of CRISPR/Cas9 to reduce the risk of cardiovascular diseases. Angew Chem Int Edit. 2019;58:12404–8.
Chen X, Chen Y, Xin H, Wan T, Ping Y. Near-infrared optogenetic engineering of photothermal nanoCRISPR for programmable genome editing. Proc Natl Acad Sci U S A. 2020;117:2395–405.
Peng H, Le C, Wu J, Li X, Zhang H, Le X. A Genome-editing nanomachine constructed with a clustered regularly interspaced short palindromic repeats system and activated by near-infrared illumination. ACS Nano. 2020;14:2817–26.
Pan Y, Yang J, Luan X, Liu X, Li X, Yang J, et al. Near-infrared upconversion–activated CRISPR-Cas9 system: a remote-controlled gene editing platform. Sci Adv. 2019;5(4):eaav7199. https://doi.org/10.1126/sciadv.aav7199.
Wu Y, Zheng J, Zeng Q, Zhang T, Xing D. Light-responsive charge-reversal nanovector for high-efficiency in vivo CRISPR/Cas9 gene editing with controllable location and time. Nano Res. 2020;13:2399–406.
Kaushik A, Yndart A, Atluri V, Tiwari S, Tomitaka A, Gupta P, et al. Magnetically guided non-invasive CRISPR-Cas9/gRNA delivery across blood-brain barrier to eradicate latent HIV-1 infection. Sci Rep-UK. 2019;9(1). https://doi.org/10.1038/s41598-019-40222-4.
Ryu J, Won E, Lee H, Kim J, Hui E, Kim H, et al. Ultrasound-activated particles as CRISPR/Cas9 delivery system for androgenic alopecia therapy. Biomaterials. 2020;232:119736. https://doi.org/10.1016/j.biomaterials.2019.119736.
Trabulo S, Cardoso A, Düzgüneş N, Jurado A, Pedroso de Lima M. Cell-penetrating peptide-based systems for nucleic acid delivery: a biological and biophysical approach. Method Enzymol. 2012;509:277–300. https://doi.org/10.1016/B978-0-12-391858-1.00014-9.
Hoffner G, Soues S, Djian P. Aggregation of expanded huntingtin in the brains of patients with Huntington disease. Prion. 2007;1:26–31.
Shin J, Kim K, Chao M, Atwal R, Gillis T, MacDonald M, et al. Permanent inactivation of Huntington’s disease mutation by personalized allele-specific CRISPR/Cas9. Hum Mol Genet. 2016:ddw286. https://doi.org/10.1093/hmg/ddw286.
Monteys A, Ebanks S, Keiser M, Davidson B. CRISPR/Cas9 editing of the mutant Huntingtin allele in vitro and in vivo. Mol Ther. 2017;25:12–23.
Gasiunas G, Barrangou R, Horvath P, Siksnys V. Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc Natl Acad Sci U S A. 2012;109:E2579–86. https://doi.org/10.1073/pnas.1208507109.
Dabrowska M, Juzwa W, Krzyzosiak W, Olejniczak M. Precise excision of the CAG tract from the Huntingtin gene by Cas9 nickases. Front Neurosci-Switz. 2018;12. https://doi.org/10.3389/fnins.2018.00075.
Jiang H, Mankodi A, Swanson M, Moxley R, Thornton C. Myotonic dystrophy type 1 is associated with nuclear foci of mutant RNA, sequestration of muscleblind proteins and deregulated alternative splicing in neurons. Hum Mol Genet. 2004;13:3079–88.
Lin X, Miller J, Mankodi A, Kanadia R, Yuan Y, Moxley R, et al. Failure of MBNL1-dependent post-natal splicing transitions in myotonic dystrophy. Hum Mol Genet. 2006;15:2087–97.
Dastidar S, Ardui S, Singh K, Majumdar D, Nair N, Fu Y, et al. Efficient CRISPR/Cas9-mediated editing of trinucleotide repeat expansion in myotonic dystrophy patient-derived iPS and myogenic cells. Nucleic Acids Res. 2018;46:8275–98.
Lo Scrudato M, Poulard K, Sourd C, Tomé S, Klein A, Corre G, et al. Genome editing of expanded CTG repeats within the human DMPK gene reduces nuclear RNA foci in the muscle of DM1 mice. Mol Ther. 2019;27:1372–88.
Wang Y, Hao L, Wang H, Santostefano K, Thapa A, Cleary J, et al. Therapeutic genome editing for myotonic dystrophy type 1 using CRISPR/Cas9. Mol Ther. 2018;26:2617–30.
Ikeda M, Taniguchi-Ikeda M, Kato T, Shinkai Y, Tanaka S, Hagiwara H, et al. Unexpected mutations by CRISPR-Cas9 CTG repeat excision in myotonic dystrophy and use of CRISPR interference as an alternative approach. Mol Ther Methods Clin. 2020;18:131–44.
Batra R, Nelles D, Pirie E, Blue S, Marina R, Wang H, et al. Elimination of toxic microsatellite repeat expansion RNA by RNA-targeting Cas9. Cell. 2017;170(5):899–912.e10.
Batra R, Nelles D, Roth D, Krach F, Nutter C, Tadokoro T, et al. The sustained expression of Cas9 targeting toxic RNAs reverses disease phenotypes in mouse models of myotonic dystrophy type 1. Nat Biomed Eng. 2020;5:157–68. https://doi.org/10.1038/s41551-020-00607-7.
Castaman G, Matino D. Hemophilia A and B: molecular and clinical similarities and differences. Haematologica. 2019;104:1702–9.
Sung J, Park C, Leem J, Cho M, Kim D. Restoration of FVIII expression by targeted gene insertion in the FVIII locus in hemophilia A patient-derived iPSCs. Exp Mol Med. 2019;51:1–9.
Chen H, Shi M, Gilam A, Zheng Q, Zhang Y, Afrikanova I, et al. Hemophilia A ameliorated in mice by CRISPR-based in vivo genome editing of human Factor VIII. Sci Rep-UK. 2019;9(1). https://doi.org/10.1038/s41598-019-53198-y.
Park C, Sung J, Cho S, Kim J, Kim D. Universal correction of blood coagulation factor VIII in patient-derived induced pluripotent stem cells using CRISPR/Cas9. Stem Cell Rep. 2019;12:1242–9.
Ohmori T, Nagao Y, Mizukami H, Sakata A, Muramatsu S, Ozawa K, et al. CRISPR/Cas9-mediated genome editing via postnatal administration of AAV vector cures hemophilia B mice. Sci Rep-UK. 2017;7(1). https://doi.org/10.1038/s41598-017-04625-5.
Wang L, Yang Y, Breton C, White J, Zhang J, Che Y, et al. CRISPR/Cas9-mediated in vivo gene targeting corrects hemostasis in newborn and adult factor IX–knockout mice. Blood. 2019;133:2745–52.
Babačić H, Mehta A, Merkel O, Schoser B. CRISPR-cas gene-editing as plausible treatment of neuromuscular and nucleotide-repeat-expansion diseases: a systematic review. PLoS One. 2019;14(2):e0212198. https://doi.org/10.1371/journal.pone.0212198.
Shimo T, Maruyama R, Yokota T. Designing effective antisense oligonucleotides for exon skipping. Methods Mol Biol. 2017:143–55.
Aartsma-Rus A, Straub V, Hemmings R, Haas M, Schlosser-Weber G, Stoyanova-Beninska V, et al. Development of exon skipping therapies for duchenne muscular dystrophy: a critical review and a perspective on the outstanding issues. Nucleic Acid Ther. 2017;27:251–9.
Amoasii L, Long C, Li H, Mireault A, Shelton J, Sanchez-Ortiz E, et al. Single-cut genome editing restores dystrophin expression in a new mouse model of muscular dystrophy. Sci Transl Med. 2017;9(418):eaan8081. https://doi.org/10.1126/scitranslmed.aan8081.
Amoasii L, Hildyard J, Li H, Sanchez-Ortiz E, Mireault A, Caballero D, et al. Gene editing restores dystrophin expression in a canine model of Duchenne muscular dystrophy. Science. 2018;362:86–91.
Ifuku M, Iwabuchi K, Tanaka M, Lung M, Hotta A. Restoration of dystrophin protein expression by exon skipping utilizing CRISPR-Cas9 in myoblasts derived from DMD patient iPS Cells. Methods Mol Biol. 1828;2018:191–217.
Min Y, Li H, Rodriguez-Caycedo C, Mireault A, Huang J, Shelton J, et al. CRISPR-Cas9 corrects Duchenne muscular dystrophy exon 44 deletion mutations in mice and human cells. Sci Adv. 2019;5(3):eaav4324. https://doi.org/10.1126/sciadv.aav4324.
Hamel C. Retinitis pigmentosa. Orphanet J Rare Dis. 2006;1(1). https://doi.org/10.1186/1750-1172-1-40.
Daiger S, Sullivan L, Bowne S. Genes and mutations causing retinitis pigmentosa. Clin Genet. 2013;84:132–41.
Athanasiou D, Aguila M, Bellingham J, Li W, McCulley C, Reeves P, et al. The molecular and cellular basis of rhodopsin retinitis pigmentosa reveals potential strategies for therapy. Prog Retin Eye Res. 2018;62:1–23.
Tsai Y, Wu W, Lee T, Wu W, Xu C, Park K, et al. Clustered regularly interspaced short palindromic repeats-based genome surgery for the treatment of autosomal dominant retinitis pigmentosa. Ophthalmology. 2018;125:1421–30.
Vagni P, Perlini L, Chenais N, Marchetti T, Parrini M, Contestabile A, et al. Gene editing preserves visual functions in a mouse model of retinal degeneration. Front Neurosci-Switz. 2019;13. https://doi.org/10.3389/fnins.2019.00945.
Cai Y, Cheng T, Yao Y, Li X, Ma Y, Li L, et al. In vivo genome editing rescues photoreceptor degeneration via a Cas9/RecA-mediated homology-directed repair pathway. Sci Adv. 2019;5(4):eaav3335. https://doi.org/10.1126/sciadv.aav3335.
Cremers F. Molecular genetics of Leber congenital amaurosis. Hum Mol Genet. 2002;11:1169–76.
Maeder M, Shen S, Burnight E, Gloskowski S, Mepani R, Friedland A, et al. 687. Therapeutic correction of an LCA-causing splice defect in the CEP290 gene by CRISPR/Cas-mediated genome editing. Mol Ther. 2015;23:S273–4.
Burnight E, Wiley L, Drack A, et al. CEP290 gene transfer rescues Leber congenital amaurosis cellular phenotype. Gene Ther. 2014;21:662–72. https://doi.org/10.1038/gt.2014.39.
Maeder M, Stefanidakis M, Wilson C, Baral R, Barrera L, Bounoutas G, et al. Development of a gene-editing approach to restore vision loss in Leber congenital amaurosis type 10. Nat Med. 2019;25:229–33.
MA B. Sickle Cell Disease [Internet]. GeneReviews®. 2003 [cited 27 February 2021]. Available from: https://pubmed.ncbi.nlm.nih.gov/20301551/
Ashley-Koch A, Yang Q, Olney R. Sickle hemoglobin (Hb S) allele and sickle Cell disease: a HuGE review. Am J Epidemiol. 2000;151:839–45.
Thein S. The molecular basis of β-thalassemia. CSH Perspect Med. 2013;3(5):a011700–0.
Cai L, Bai H, Mahairaki V, Gao Y, He C, Wen Y, et al. A universal approach to correct various HBB gene mutations in human stem cells for gene therapy of ®-thalassemia and sickle cell disease. Stem Cells Transl Med. 2017;7:87–97.
Basak A, Hancarova M, Ulirsch J, Balci T, Trkova M, Pelisek M, et al. BCL11A deletions result in fetal hemoglobin persistence and neurodevelopmental alterations. J Clin Invest. 2015;125:2363–8.
GlobeNewswire: CRISPR Therapeutics and Vertex Announce New Clinical Data for Investigational Gene-Editing Therapy CTX001TM in Severe Hemoglobinopathies at the 25th Annual European Hematology Association (EHA) Congress [Internet]. Globenewswire.com. 2020 [cited 30 August 2020]. Available from: https://www.globenewswire.com/news-release/2020/06/12/2047260/0/en/CRISPR-Therapeutics-and-Vertex-Announce-New-Clinical-Data-for-Investigational-Gene-Editing-Therapy-CTX001-in-Severe-Hemoglobinopathies-at-the-25th-Annual-European-Hematology-Associ.html
Chen Y, Sheng J, Trang P, Liu F. Potential application of the CRISPR/Cas9 system against herpesvirus infections. Viruses. 2018;10(6):291. https://doi.org/10.3390/v10060291.
van Diemen F, Kruse E, Hooykaas M, Bruggeling C, Schürch A, van Ham P, et al. CRISPR/Cas9-mediated genome editing of herpesviruses limits productive and latent infections. PLoS Pathog. 2016;12(6):e1005701. https://doi.org/10.1371/journal.ppat.1005701.
Yin D, Ling S, Wang D, Dai Y, Jiang H, Zhou X, et al. Targeting herpes simplex virus with CRISPR–Cas9 cures herpetic stromal keratitis in mice. Nat Biotechnol. 2021. https://doi.org/10.1038/s41587-020-00781-8.
Okoye A, Picker L. CD4+T-cell depletion in HIV infection: mechanisms of immunological failure. Immunol Rev. 2013;254:54–64.
Dahabieh M, Battivelli E, Verdin E. Understanding HIV latency: the road to an HIV cure. Annu Rev Med. 2015;66:407–21.
Liao H, Gu Y, Diaz A, Marlett J, Takahashi Y, Li M, et al. Use of the CRISPR/Cas9 system as an intracellular defense against HIV-1 infection in human cells. Nat Commun. 2015;6(1). https://doi.org/10.1038/ncomms7413.
Zhu W, Lei R, Le Duff Y, Li J, Guo F, Wainberg M, et al. The CRISPR/Cas9 system inactivates latent HIV-1 proviral DNA. Retrovirology. 2015;12(1). https://doi.org/10.1186/s12977-015-0150-z.
Wang G, Zhao N, Berkhout B, Das A. A combinatorial CRISPR-Cas9 attack on HIV-1 DNA extinguishes all infectious provirus in infected T cell cultures. Cell Rep. 2016;17:2819–26.
Lebbink R, de Jong D, Wolters F, Kruse E, van Ham P, Wiertz E, et al. A combinational CRISPR/Cas9 gene-editing approach can halt HIV replication and prevent viral escape. Sci Rep-UK. 2017;7(1). https://doi.org/10.1038/srep41968.
Ophinni Y, Inoue M, Kotaki T, Kameoka M. CRISPR/Cas9 system targeting regulatory genes of HIV-1 inhibits viral replication in infected T-cell cultures. Sci Rep-UK. 2018;8(1). https://doi.org/10.1038/s41598-018-26190-1.
Zhao N, Wang G, Das A, Berkhout B. Combinatorial CRISPR-Cas9 and RNA interference attack on HIV-1 DNA and RNA can lead to cross-resistance. Antimicrob Agents Ch. 2017;61(12). doi:https://doi.org/10.1128/AAC.01486-17.
Dash P, Kaminski R, Bella R, Su H, Mathews S, Ahooyi T et al. Sequential LASER ART and CRISPR treatments eliminate HIV-1 in a subset of infected humanized mice. Nat Commun. 2019;10(1). https://doi.org/10.1038/s41467-019-10366-y.
Münger K, Baldwin A, Edwards K, Hayakawa H, Nguyen C, Owens M, et al. Mechanisms of human papillomavirus-Induced oncogenesis. J Virol. 2004;78:11451–60. https://doi.org/10.1128/JVI.78.21.11451-11460.2004.
Zhen S, Lu J, Wang L, Sun X, Zhang J, Li X, et al. In vitro and in vivo synergistic therapeutic effect of cisplatin with human papillomavirus16 E6/E7 CRISPR/Cas9 on cervical cancer cell line. Transl Oncol. 2016;9:498–504.
Zhen S, Lu J, Liu Y, Chen W, Li X. Synergistic antitumor effect on cervical cancer by rational combination of PD1 blockade and CRISPR-Cas9-mediated HPV knockout. Cancer Gene Ther. 2019;27:168–78.
Ling K, Yang L, Yang N, Chen M, Wang Y, Liang S, et al. Gene targeting of HPV18 E6 and E7 synchronously by nonviral transfection of CRISPR/Cas9 system in cervical cancer. Hum Gene Ther. 2020;31:297–308.
Tu T, Budzinska M, Vondran F, Shackel N, Urban S. Hepatitis B virus DNA integration occurs early in the viral life cycle in an in vitro infection model via sodium taurocholate cotransporting polypeptide-dependent uptake of enveloped virus particles. J Virol. 2018;92(11):e02007–17. https://doi.org/10.1128/JVI.02007-17.
Werle-Lapostolle B, Bowden S, Locarnini S, Wursthorn K, Petersen J, Lau G, et al. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology. 2004;126:1750–8. https://doi.org/10.1053/j.gastro.2004.03.018.
Liu X, Hao R, Chen S, Guo D, Chen Y. Inhibition of hepatitis B virus by the CRISPR/Cas9 system via targeting the conserved regions of the viral genome. J Gen Virol. 2015;96(8):2252–61. https://doi.org/10.1099/vir.0.000159.
Karimova M, Beschorner N, Dammermann W, Chemnitz J, Indenbirken D, Bockmann J et al. CRISPR/Cas9 nickase-mediated disruption of hepatitis B virus open reading frame S and X. Sci Rep-UK. 2015;5(1). https://doi.org/10.1038/srep13734.
Dong C, Qu L, Wang H, Wei L, Dong Y, Xiong S. Targeting hepatitis B virus cccDNA by CRISPR/Cas9 nuclease efficiently inhibits viral replication. Antivir Res. 2015;118:110–7. https://doi.org/10.1016/j.antiviral.2015.03.015.
Sakuma T, Masaki K, Abe-Chayama H, Mochida K, Yamamoto T, Chayama K. Highly multiplexed CRISPR-Cas9-nuclease and Cas9-nickase vectors for inactivation of hepatitis B virus. Genes Cells. 2016;21:1253–62. https://doi.org/10.1111/gtc.12437.
Li H, Sheng C, Liu H, Wang S, Zhao J, Yang L, et al. Inhibition of HBV expression in HBV transgenic mice using AAV-delivered CRISPR-SaCas9. Front Immunol. 2018;9. https://doi.org/10.3389/fimmu.2018.02080.
Liu Y, Zhao M, Gong M, Xu Y, Xie C, Deng H, et al. Inhibition of hepatitis B virus replication via HBV DNA cleavage by Cas9 from Staphylococcus aureus. Antivir Res. 2018;152:58–67.
Stone D, Long K, Loprieno M, De Silva FH, Kenkel E, Liley R, et al. CRISPR-Cas9 gene editing of hepatitis B virus in chronically infected humanized mice. Mol Ther Methods Clin. 2020;20:258–75.
Kayesh M, Amako Y, Hashem M, Murakami S, Ogawa S, Yamamoto N, et al. Development of an in vivo delivery system for CRISPR/Cas9-mediated targeting of hepatitis B virus cccDNA. Virus Res. 2020;290:198191. https://doi.org/10.1016/j.virusres.2020.198191.
Zhen S, Qiang R, Lu J, Tuo X, Yang X, Li X. Enhanced antiviral benefit of combination therapy with anti-HBV and anti-PD1 gRNA/cas9 produces a synergistic antiviral effect in HBV infection. Mol Immunol. 2021;130:7–13.
Lu Y, Xue J, Deng T, Zhou X, Yu K, Deng L, et al. Safety and feasibility of CRISPR-edited T cells in patients with refractory non-small-cell lung cancer. Nat Med. 2020;26:732–40.
Wang X, Rivière I. Clinical manufacturing of CAR T cells: foundation of a promising therapy. Mol Ther-Oncolytics. 2016;3:16015. https://doi.org/10.1038/mto.2016.15.
Depil S, Duchateau P, Grupp S, Mufti G, Poirot L. ‘Off-the-shelf’ allogeneic CAR T cells: development and challenges. Nat Rev Drug Discov. 2020;19:185–99. https://doi.org/10.1038/s41573-019-0051-2.
CRISPR Therapeutics Reports Positive Top-Line Results from Its Phase 1 CARBON Trial of CTX110™ in Relapsed or Refractory CD19+ B-cell Malignancies. GlobeNewswire News Room. https://www.globenewswire.com/news-release/2020/10/21/2111729/0/en/CRISPR-Therapeutics-Reports-Positive-Top-Line-Results-from-Its-Phase-1-CARBON-Trial-of-CTX110-in-Relapsed-or-Refractory-CD19-B-cell-Malignancies.html. Published 2021. Accessed March 14, 2021.
Ayanoglu F, Elcin A, Elcin Y. Bioethical issues in genome editing by CRISPR-Cas9 technology. Turk J Biol. 2020;44(2):110–20.
Yang H, Ren S, Yu S, Pan H, Li T, Ge S, et al. Methods favoring homology-directed repair choice in response to CRISPR/Cas9 induced-double strand breaks. Int J Mol Sci. 2020;21(18):6461. https://doi.org/10.3390/ijms21186461.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ramirez-Phillips, A.C., Liu, D. Therapeutic Genome Editing and In Vivo Delivery. AAPS J 23, 80 (2021). https://doi.org/10.1208/s12248-021-00613-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-021-00613-w