Abstract
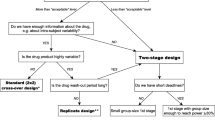
This paper introduces a two-stage bioequivalence design involving the selection of one out of two candidate formulations at an initial stage and quantifies the overall power (chance of ultimately showing bioequivalence) in a range of scenarios with CVs ranging from 0.1 to 1. The methods introduced are derivates of the methods invented in 2008 by Diane Potvin and co-workers (Pharm Stat. 7(4): 245-262, 2008). The idea is to test the two candidate formulations independently in an initial stage, making a selection of one of these formulations basis of the observed point estimates, and to run, when necessary, a second stage of the trial with pooling of data. Alpha levels are identified which are shown to control the maximum type I error at 5%. Results, expressed as powers and sample sizes, are also published for scenarios where the two formulations are far apart in terms of the match against the reference (one GMR being 0.80, the other GMR being 0.95) and in scenarios where the two test formulations have an actual better match (one GMR being 0.90, the other GMR being 0.95). The methods seem to be compliant with wording of present guidelines from EMA, FDA, WHO, and Health Canada. Therefore the work presented here may be useful for companies developing drugs whose approval hinges on in vivo proof of bioequivalence and where traditional in vitro screening methods (such as dissolution trials) may have poor ability to predict the best candidate.



Similar content being viewed by others
References
Potvin D, DiLiberti CE, Hauck WW, Parr AF, Schuirmann DJ, Smith RA. Sequential design approaches for bioequivalence studies with crossover designs. Pharm Stat. 2008;7(4):245–62.
Montague TH, Potvin D, Diliberti CE, Hauck WW, Parr AF, Schuirmann DJ. Additional results for ‘Sequential design approaches for bioequivalence studies with crossover designs’. Pharm Stat. 2012;11(1):8–13.
Karalis V, Macheras P. An insight into the properties of a two-stage design in bioequivalence studies. Pharm Res. 2013;30(7):1824–35.
Maurer W, Jones B, Chen Y. Controlling the type I error rate in two-stage sequential adaptive designs when testing for average bioequivalence. Stat Med. 37(10):1587–607.
Kieser M, Rauch G. Two-stage designs for cross-over bioequivalence trials. Stat Med. 2015;34(16):2403–16.
Wonnemann M, Frömke C, Koch A. Inflation of the type I error: investigations on regulatory recommendations for bioequivalence of highly variable drugs. Pharm Res. 2015;32(1):135–43.
Karalis V. The role of the upper sample size limit in two-stage bioequivalence designs. Int J Pharm. 2013;456(1):87–94.
Karalis V, Macheras P. On the statistical model of the two-stage designs in bioequivalence assessment. J Pharm Pharmacol. 2014;66(1):48–52.
Fuglsang A. Futility rules in bioequivalence trials with sequential designs. AAPS J. 2014;16(1):79–82.
Fuglsang A. Sequential bioequivalence approaches for parallel designs. AAPS J. 2014;16(3):373–8.
Fuglsang A. A sequential bioequivalence design with a potential ethical advantage. AAPS J. 2014;6(4):843–6.
Fuglsang A. Sequential bioequivalence trial designs with increased power and controlled type I error rates. AAPS J. 2013;15(3):659–61.
Xu J, Audet C, DiLiberti CE, Hauck WW, Montague TH, Parr AF, et al. Optimal adaptive sequential designs for crossover bioequivalence studies. Pharm Stat. 2016;15(1):15–27.
Schütz H. Two-stage designs in bioequivalence trials. Eur J Clin Pharmacol. 2015;71(3):271–81.
United States Food and Drug Administration, Center for Drug Evaluation and Research. Bioequivalence Studies With Pharmacokinetic Endpoints for Drugs Submitted Under an Abbreviated New Drug Application. 2013. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioequivalence-studies-pharmacokinetic-endpoints-drugs-submitted-under-abbreviated-new-drug.
United States Food and Drug Administration, Center for Drug Evaluation and Research. Statistical Approaches to Establishing Bioequivalence. Guidance for Industry: Statistical Approaches to Establishing Bioequivalence. 2001. http://www.fda.gov/downloads/Drugs/Guidances/ucm070244.pdf.
Committee for Human Medicinal Products. Investigation of bioequivalence. CHMP. CPMP/EWP/QWP/1401/98 Rev. 1. 2010. http://www.ema.europa.eu/ema/pages/includes/document/open_document.jsp?webContentId=WC500070039. Accessed 11 April 2020.
Therapeutic Products Directorate, Health Canada. Conduct and Analysis of Comparative Bioavailability Studies. 2012. http://www.hc-sc.gc.ca/dhp-mps/alt_formats/pdf/prodpharma/applic-demande/guide-ld/bio/gd_cbs_ebc_ld-eng.pdf. Accessed 11 April 2020.
World Health Organization. Annex 7, Multisource (generic) pharmaceutical products: guidelines on registration requirements to establish interchangeability. WHO Technical Report Series No. 992, 2015. https://www.who.int/medicines/areas/quality_safety/quality_assurance/Annex7-TRS992.pdf. Accessed 11 April 2020.
Matsumoto M, Nishimura T. Mersenne twister: a 623-dimensionally equidistributed uniform pseudorandom number generator. ACM Trans Model Comput Simul. 1998;8(1):3–30.
Burkhardt J. CDF of the noncentral T Distribution. https://people.sc.fsu.edu/~jburkardt/c_src/asa243/asa243.html. Accessed 11 April 2020.
Marsaglia G, Bray TA. A convenient method for generating normal variables. SIAM Rev. 1964;6(3):260–4.
Fuglsang A. Controlling type I errors for two-stage bioequivalence study designs. Clin Res Regul Aff. 2011;28:100–5.
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. Statistical Principles for Clinical Trials E9. 1998. https://database.ich.org/sites/default/files/E9_Guideline.pdf. Accessed 11 April 2020.
Davit BM, Conner DP, Fabian-Fritsch B, Haidar SH, Jiang X, Patel DT, et al. Highly variable drugs: observations from bioequivalence data submitted to the FDA for new generic drug applications. AAPS J. 2008;10(1):148–56.
Molins E, Cobo E, Ocaña J. Two-stage designs versus European scaled average designs in bioequivalence studies for highly variable drugs: which to choose? Stat Med. 2017;36(30):4777–88.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares no conflict of interest.
Author Disclosure
The author is a consultant. Present and former clients include WHO, USP, agencies, and private companies.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 1803 kb)
Rights and permissions
About this article
Cite this article
Fuglsang, A. A Three-Treatment Two-Stage Design for Selection of a Candidate Formulation and Subsequent Demonstration of Bioequivalence. AAPS J 22, 109 (2020). https://doi.org/10.1208/s12248-020-00492-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-020-00492-7