Abstract
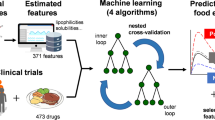
Drug absorption is a complex process governed by a number of interrelated physicochemical, biopharmaceutical, and pharmacokinetic factors. In order to explore complex relationships among these factors, multivariate exploratory analysis was performed on the dataset of drugs with diverse bioperformance. The investigated dataset included subset of drugs for which bioequivalence between solid dosage form and oral solution has been reported, and subset of drugs described in the literature as low solubility/low permeability compounds. Discriminatory power of hierarchical clustering on principal components was somewhat higher when applied on the data subsets of drugs with similar bioperformance, while analysis of the integrated dataset indicated existence of two groups of drugs with the boundaries reflected in Peff value of approximately 2 × 10−4 cm/s and Fa and Fm values higher than 85% and 50%, respectively. Majority of the investigated drugs within the integrated dataset were grouped within their initial subset indicating that overall drug bioperformance is closely related to its physicochemical, biopharmaceutical and pharmacokinetic properties. Classification models constructed using the random forest (RF) and support vector machine with polynomial kernel function were able to predict food effect based on drug dose/solubility ratio (D/S), effective permeability (Peff), percent of dose metabolized (Fm), and elimination half-life (τ1/2). Although both models performed well during training and testing, only RF kept satisfying performance when applied on the external dataset (kappa value > 0.4). The results obtained indicate that data mining can be employed as useful tool in biopharmaceutical drug characterization which merits further investigation.



Similar content being viewed by others
References
Caspari C. A Treatise on pharmacy for students and pharmacists.Philadelphia: Lea Brothers & Company; 1895.
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 1997;23(1-3):3–25.
Amidon GL, Lennernas H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12(3):413–20.
Burton PS, Goodwin JT, Vidmar TJ, Amore BM. Predicting drug absorption: how nature made it a difficult problem. J Pharmacol Exp Ther. 2002;303(3):889–95.
Kaplan S. Biological implications of in vitro dissolution testing. In: Leeson L, Cartensen JT, editors. Dissolution Technology. Washington DC: The Industrial Pharmaceutical Technology Section of Pharmaceutical Sciences; 1974. p. 167.
Sugano K. Theoretical investigation of dissolution test criteria for waiver of clinical bioequivalence study. J Pharm Sci. 2016;105(6):1947–51.
Lennernas H. Intestinal permeability and its relevance for absorption and elimination. Xenobiotica. 2007;37(10-11):1015–51.
Dahan A, Miller JM. The solubility–permeability interplay and its implications in formulation design and development for poorly soluble drugs. AAPS J. 2012;14(2):244–51.
Butler J, Hens B, Vertzoni M, Brouwers J, Berben P, Dressman J, et al. In vitro models for the prediction of in vivo performance of oral dosage forms: recent progress from partnership through the IMI OrBiTo collaboration. Eur J Pharm Biopharm. 2019;136:70–83.
Vertzoni M, Augustijns P, Grimm M, Koziolek M, Lemmens G, Parrott N, et al. Impact of regional differences along the gastrointestinal tract of healthy adults on oral drug absorption: An UNGAP review. Eur J Pharmacol. 2019;134:153–75.
Koziolek M, Alcaro S, Augustijns P, Basit AW, Grimm M, Hens B, et al. The mechanisms of pharmacokinetic food-drug interactions–a perspective from the UNGAP group. Eur J Pharmacol. 2019;134:31–59.
Ghadi R, Dand N. BCS class IV drugs: highly notorious candidates for formulation development. J Control Release. 2017;248:71–95.
Martinez MN, Amidon GL. A mechanistic approach to understanding the factors affecting drug absorption: a review of fundamentals. J Clin Pharmacol. 2002;42(6):620–43.
Wu C-Y, Benet LZ. Predicting drug disposition via application of BCS: transport/absorption/elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm Res. 2005;22(1):11–23.
Pham-The H, Garrigues T, Bermejo M, Gonzalez-Alvarez I, Monteagudo MC, Cabrera-Perez MA. Provisional classification and in silico study of biopharmaceutical system based on caco-2 cell permeability and dose number. Mol Pharm. 2013;10(6):2445–61.
Newby D, Freitas AA, Ghafourian T. Comparing multilabel classification methods for provisional biopharmaceutics class prediction. Mol Pharm. 2014;12(1):87–102.
Chatzizacharia K, Hatziavramidis D. New frames of reference for mapping drugs in the four classes of the BCS and BDDCS into regions with clear boundaries. AICHE J. 2015;61(11):3570–9.
Daousani C, Macheras P, Karalis V.Understanding the linkage between pharmacokinetic properties and the two classification systems: BCS and BDDCS. AAPS Annual Meeting and Exposition 2016; Denver, CO.
Gatarić B, Parojčić J. Application of data mining approach to identify drug subclasses based on solubility and permeability. Biopharm Drug Dispos. 2019;40(2):51–61.
Kim S, Chen J, Cheng T, Gindulyte A, He J, He S, et al. PubChem 2019 update: improved access to chemical data. Nucleic Acids Res. 2018;47(D1):D1102–D9.
Ursu O, Holmes J, Knockel J, Bologa CG, Yang JJ, Mathias SL, et al. DrugCentral: online drug compendium. Nucleic Acids Res. 2016;45(D):D932–9.
Newby D, Freitas AA, Ghafourian T. Decision trees to characterise the roles of permeability and solubility on the prediction of oral absorption. Eur J Med Chem. 2015;90:751–65.
Benet LZ, Hosey CM, Ursu O, Oprea TI. BDDCS, the rule of 5 and drugability. Adv Drug Deliv Rev. 2016;101:89–98.
Brune K.Pharmacokinetics of azapropazone in comparison to other NSAIDs.In: Rainsford KD, editor. Azapropazone.Dordrecht: Kluwer Academic;1989.p.53-61.
Larregieu C, Benet L. Distinguishing between the permeability relationships with absorption and metabolism to improve BCS and BDDCS predictions in early drug discovery. Mol Pharm. 2014;11(4):1335–44.
Beermann B, Groschinsky-Grind M. Clinical pharmacokinetics of diuretics. Clin Pharmacokinet. 1980;5(3):221–45.
Rinaki E, Valsami G, Macheras P. Quantitative biopharmaceutics classification system: The central role of dose/solubility ratio. Pharm Res. 2003;20(12):1917–25.
Rinaki E, Dokoumetzidis A, Macheras P. The mean dissolution time depends on the dose/solubility ratio. Pharm Res. 2003;20(3):406–8.
Macheras P, Karalis V, Valsami G. Keeping a critical eye on the science and the regulation of oral drug absorption: a review. J Pharm Sci. 2013;102(9):3018–36.
Jolliffe IT. Principal component analysis. 2005.
Husson F, Josse J, Pages J. Principal component methods-hierarchical clustering-partitional clustering: why would we need to choose for visualizing data?.In: Technical report-Agro campus. Applied Mathematics Department. 2010. http://factominer.free.fr/more/HCPC_husson_josse.pdf. Accessed 20 Aug 2019.
Lê S, Josse J, Husson F. FactoMineR: an R package for multivariate analysis. J Stat Softw. 2008;25(1):1–18.
Kassambara A, Mundt F. Factoextra: extract and visualize the results of multivariate data analyses. R package version 1.0. 5., 2017.
Kuhn M, Wind J, Weston S, Williams A, Keefer C, Engelhardt A, et al. caret: Classification and regression training. R package ver. 6.0–84. 2019.
Branco P, Ribeiro RP, Torgo L. UBL: an R package for utility-based learning. arXiv preprint arXiv:160408079. 2016.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics.1977;33(1):159–74.
Kuhn M. Variable importance using the caret package. 2012.
Ohashi R, Watanabe R, Esaki T, Taniguchi T, Torimoto-Katori N, et al. Development of simplified in vitro P-glycoprotein substrate assay and in silico prediction models to evaluate transport potential of P-glycoprotein. Mol Pharm. 2019;16(5):1851–63.
Sakiyama Y, Yuki H, Moriya T, Hattori K, Suzuki M, Shimada K, et al. Predicting human liver microsomal stability with machine learning techniques. J Mol Graph Model. 2008;26(6):907–15.
Chen M-L, Yu L. The use of drug metabolism for prediction of intestinal permeability. Mol Pharm. 2009;6(1):74–81.
Macheras P, Karalis V. A non-binary biopharmaceutical classification of drugs: the ABΓ system. Int J Pharm. 2014;464(1-2):85–90.
Gu C-H, Li H, Levons J, Lentz K, Gandhi RB, Raghavan K, et al. Predicting effect of food on extent of drug absorption based on physicochemical properties. Pharm Res. 2007;24(6):1118–30.
Li M, Zhao P, Pan Y, Wagner C. Predictive performance of physiologically based pharmacokinetic models for the effect of food on oral drug absorption: current status. CPT Pharmacometrics Syst Pharmacol. 2018;7(2):82–9.
Custodio JM, Wu C-Y, Benet LZ. Predicting drug disposition, absorption/elimination/transporter interplay and the role of food on drug absorption. Adv Drug Deliv Rev. 2008;60(6):717–33.
Fleisher D, Li C, Zhou Y, Pao LH, Karim A. Drug, Meal and formulation interactions infulencing drug absorpion after oral administration. Clin Pharmacokinet. 1999;36(3):233–54.
Carver PL, Fleisher D, Zhou SY, Kaul D, Kazanjian P, Li C. Meal composition effects on the oral bioavailability of indinavir in HIV-infected patients. Pharm Res. 1999;16(5):718–24.
Pabla D, Akhlaghi F, Zia H. Intestinal permeability enhancement of levothyroxine sodium by straight chain fatty acids studied in MDCK epithelial cell line. Eur J Pharmacol. 2010;40(5):466–72.
Liedholm H, Melander A. Concomitant food intake can increase the bioavailability of propranolol by transient inhibition of its presystemic primary conjugation. Clin Pharmacol Ther. 1986;40(1):29–36.
Melander A, Danielson K, Schersten B, Wåhlin E. Enhancement of the bioavailability of propranolol and metoprolol by food. Clin Pharmacol Ther. 1977;22(1):108–12.
Butler JM, Dressman JB. The developability classification system: application of biopharmaceutics concepts to formulation development. J Pharm Sci. 2010;99(12):4940–54.
Williams L, Davis JA, Lowenthal DT. The influence of food on the absorption and metabolism of drugs. Med Clin North Am. 1993;77(4):815–29.
Beermann B, Groschinsky-Grind M. Gastrointestinal absorption of hydrochlorothiazide enhanced by concomitant intake of food. Eur J Clin Pharmacol. 1978;13(2):125–8.
Barbhaiya RH, Craig WA, Corrick-West HP, Welling PG. Pharmacokinetics of hydrochlorothiazide in fasted and nonfasted subjects: a comparison of plasma level and urinary excretion methods. J Pharm Sci. 1982;71(2):245–8.
Funding
This work was supported by the project no. TR 34007, funded by the Ministry of Education, Science and Technological Development, Republic of Serbia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gatarić, B., Parojčić, J. An Investigation into the Factors Governing Drug Absorption and Food Effect Prediction Based on Data Mining Methodology. AAPS J 22, 11 (2020). https://doi.org/10.1208/s12248-019-0394-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-019-0394-y