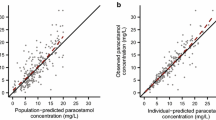
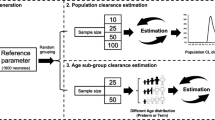
Abstract
Body weight is the primary covariate in pharmacokinetics of many drugs and dramatically changes during the first weeks of life of neonates. The objective of this study is to determine if missing body weights in preterm and term neonates affect estimates of model parameters and which methods can be used to improve performance of a population pharmacokinetic model of paracetamol. Data for our analysis were obtained from previously published studies on the pharmacokinetics of intravenous paracetamol in neonates. We adopted a population model of body weight change in neonates to implement three previously introduced methods of handling missing covariates based on data imputation, likelihood function modification, and full random effects modeling. All models were implemented in NONMEM 7.4, and population parameters were estimated using the FOCE method. Our major finding was that missing body weights minimally affect population estimates of pharmacokinetic parameters but do affect the covariate relationship parameters, particularly the one describing dependence of clearance on body weight. None of the tested methods changed estimates of between-subject variability nor impacted the predictive performance of the model. Our analysis shows that a modeling approach towards handling missing covariates allows borrowing information gathered in various studies as long as they target the same population. This approach is particularly useful for handling time-dependent missing covariates.




Similar content being viewed by others
References
Wahlby U, Thomson AH, Milligan PA, Karlsson MO. Models for time-varying covariates in population pharmacokinetic-pharmacodynamic analysis. Br J Clin Pharmacol. 2004;58:367–77.
Abduljalil K, Jamei M, Rostami-Hodjegan A, Johnson TN. Changes in individual drug-independent system parameters during virtual paediatric pharmacokinetic trials: introducing time-varying physiology into a paediatric PBPK model. AAPS J. 2014;16:568–76.
Boss GR, Seegmiller JE. Age-related physiological changes and their clinical significance. West J Med. 1981;135:434–40.
Anderson BJ, Allegaert K, Van den Anker JN, Cossey V, Holford NHG. Vancomycin pharmacokinetics in preterm neonates and the prediction of adult clearance. Br J Clin Pharmacol. 2007;63:75–84.
De Wildt SN. Profound changes in drug metabolism enzymes and possible effects on drug therapy in neonates and children. Expert Opin Drug Metab Toxicol. 2011;7:935–48.
Bhutani VK, Wong RJ, Vreman HJ, Stevenson DK. Bilirubin production and hour-specific bilirubin levels. J Perinatol. 2015;35:735–8.
Allegaert K, Vanhaesebrouck S, Verbesselt R, van den Anker JN. In vivo glucuronidation activity of drugs in neonates: extensive interindividual variability despite their young age. Ther Drug Monit. 2009;31:411–5.
MacDonald PD, Ross SR, Grant L, Young D. Neonatal weight loss in breast and formula fed infants. Arch Dis Child Fetal Neonatal Ed. 2003;88:F472–6.
Yang WC, Zhao LL, Li YC, Chen CH, Chang YJ, Fu YC, et al. Bodyweight loss in predicting neonatal hyperbilirubinemia 72 hours after birth in term newborn infants. BMC Pediatr. 2013;13:145.
Wilbaux M, Kasser S, Wellmann S, Lapaire O, van den Anker JN, Pfister M. Characterizing and forecasting individual weight changes in term neonates. J Pediatr. 2016;173:101–7.
Wilbaux M, Kasser S, Gromann J, Mancino I, Coscia T, Lapaire O, et al. Personalized weight change prediction in the first week of life. Clin Nutr. 2018;38:689–96. https://doi.org/10.1016/j.clnu.2018.04.001.
Duggan ST, Scott LJ. Intravenous paracetamol (acetaminophen). Drugs. 2009;69:101–13.
Allegaert K, Palmer GM, Anderson BJ. The pharmacokinetics of intravenous paracetamol in neonates: size matters most. Arch Dis Child. 2011;96:575–80.
Cook SF, Roberts JK, Samiee-Zafarghandy S, Stockmann C, King AD, Deutsch N, et al. Population pharmacokinetics of intravenous paracetamol (acetaminophen) in preterm and term neonates: model development and external evaluation. Clin Pharmacokinet. 2016;55:107–19.
Bonate PL. Pharmacokinetic-Pharmacodynamic modeling and simulation. 2nd ed. New York: Springer; 2011.
Johansson AM, Karlsson MO. Comparison of methods for handling missing covariate data. AAPS J. 2013;15:1232–41.
Wu H, Wu L. A multiple imputation method for missing covariates in non-linear mixed-effects models with application to HIV dynamics. Stat Med. 2001;20:1755–69.
Johansson AM, Karlsson MO. Multiple imputation of missing covariates in NONMEM and evaluation of the method’s sensitivity to η-shrinkage. AAPS J. 2013;15:1035–42.
Gastonguay MR, French JL, Heitjan DF, Rogers JA, PhD AJE, Ravva P. Missing data in model-based pharmacometric applications: points to consider. J Clin Pharmacol. 2010;50:63S–74S.
Karlsson MO, Jonsson EN, Wiltse CG, Wade JR. Assumption testing in population pharmacokinetic models: illustrated with an analysis of moxonidine data from congestive heart failure patients. J Pharmacokinet Biopharm. 1998;26:207–46.
De Silva AP, Moreno-Betancur M, De Livera AM, Lee KJ, Simpson JA. A comparison of multiple imputation methods for handling missing values in longitudinal data in the presence of a time-varying covariate with a non-linear association with time: a simulation study. BMC Med Res Methodol. 2017;17:114.
Gastonguay MR. A full model estimation approach for covariate effects: inference based on clinical importance and estimation precision. AAPS J. 2004;6(S1):Abstract W4354.
Karlsson M. A full model approach based on the covariance matrix of parameters and covariates. PAGE 21 (2012) Abstr 2455 [ www.page-meeting.org/?abstract=2455 ]. Accessed 6/16/2018
Zhang L, Beal SL, Sheiner LB. Simultaneous vs. sequential analysis for population PK/PD data I: best-case performance. J Pharmacokinet Pharmacodyn. 2003;30:387–404.
Allegaert K, Peeters M, Verbesselt R, Tibboel D, Naulaers G, De Hoon J, et al. Propofol pharmacokinetics in preterm and term neonates: the relevance of both postmenstrual and postnatal age. Crit Care. 2008;12(Suppl 2):P274.
Lavielle M. Mixed effects models for the population approach: models, tasks, methods, and tools. Boca Raton: CRC Press Taylor & Francis Group; 2015.
The R Project for Statistical Computing. https://www.r-project.org/. Accessed 6/16/2018
Acknowledgments
Authors acknowledge Elaine Williams from Children’s National Health System, Washington, DC, for her support in data collection.
Funding
This work was supported by a fellowship from Janssen Research & Development, a division of Janssen Pharmaceutica N.V. (WK), the Agency for Innovation by Science and Technology in Flanders (IWT) Safepedrug grant number IWT/SBO 130033 (KA, AV), and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD060543) (JvdA).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 17821 kb)
Appendices
Appendix 1. The likelihood function for IMPUT approach
Given the independence of random variables εCij, ηCLj, ηVj, the likelihood function of observing APAP plasma concentrations \( \boldsymbol{C}={\left\{{C}_{ij}\right\}}_{1\le i\le N,1\le j\le {n}_i} \) at times \( \boldsymbol{t}={\left\{{t}_{ij}\right\}}_{1\le i\le N,1\le j\le {n}_i} \) with imputed body weights \( {\boldsymbol{BW}}_{\boldsymbol{m}}={\left\{{BW}_{ij}\right\}}_{1\le i\le N,1\le j\le {m}_i} \) is of the following form (28):
where θ = (CL0, V0, BWCL, BWV) and \( \boldsymbol{\gamma} =\left({\sigma}_C^2,{\omega}_{CL}^2,{\omega}_V^2\right) \) are vectors of model parameters. The function φ describes the normal distribution
and Φ is a probability density function for the multivariate normal distribution:
and \( \boldsymbol{\Omega} =\mathbf{\operatorname{diag}}\left({\omega}_{CL}^2,{\omega}_V^2\right) \).
Appendix 2. The likelihood function for LIKELIHOOD approach
Let for subject ith 1 ≤ j ≤ mi index times for which BWs are measured and mi + 1 ≤ j ≤ ni index times for missing BWs. Also, let 1 ≤ i ≤ NV be reserved for neonates born by vaginal delivery, and NV + 1 ≤ i ≤ N for neonates born by cesarean delivery. Then, the likelihood function of observing APAP plasma concentrations becomes (26):
Here, \( \boldsymbol{BW}={\left\{{BW}_{ij}\right\}}_{1\le i\le N,1\le j\le {m}_i} \) denotes the observed BWs, and
Only model parameters θ and γ were estimated, while the fixed effect parameters for the BW model and the entries of ΩC other than \( {\omega}_{CL}^2,{\omega}_V^2 \) were fixed.
Appendix 3. The Likelihood Function for FREM Approach
The joint likelihood function for observed APAP plasma concentrations \( \boldsymbol{C}={\left\{{C}_{ij}\right\}}_{1\le i\le N,1\le j\le {n}_i} \)and observed BWs \( \boldsymbol{BW}={\left\{{BW}_{ij}\right\}}_{1\le i\le N,1\le j\le {m}_i} \)becomes:
where
and
are vectors of model parameters. Here
Only CL0, V0, BWCL, BWV, W0, BW0SEX, BW0GA, and \( {\sigma}_C^2,{\sigma}_{BW}^2,{\omega}_{CL}^2,{\omega}_V^2,{\omega}_{BW0}^2 \)were estimated, while the remaining model parameters were fixed.
Rights and permissions
About this article
Cite this article
Krzyzanski, W., Cook, S.F., Wilbaux, M. et al. Population Pharmacokinetic Modeling in the Presence of Missing Time-Dependent Covariates: Impact of Body Weight on Pharmacokinetics of Paracetamol in Neonates. AAPS J 21, 68 (2019). https://doi.org/10.1208/s12248-019-0331-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-019-0331-0