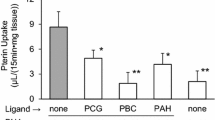
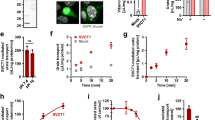
Abstract
Solute carrier organic anion transporter family member 2A1 (OATP2A1, encoded by the SLCO2A1 gene), which was initially identified as prostaglandin transporter (PGT), is expressed ubiquitously in tissues and mediates the distribution of prostanoids, such as PGE2, PGF2α, PGD2 and TxB2. It is well known to play a key role in the metabolic clearance of prostaglandins, which are taken up into the cell by OATP2A1 and then oxidatively inactivated by 15-ketoprostaglandin dehydrogenase (encoded by HPGD); indeed, OATP2A1-mediated uptake is the rate-limiting step of PGE2 catabolism. Consequently, since OATP2A1 activity is required for termination of prostaglandin signaling via prostanoid receptors, its inhibition can enhance such signaling. On the other hand, OATP2A1 can also function as an organic anion exchanger, mediating efflux of prostaglandins in exchange for import of anions such as lactate, and in this context, it plays a role in the release of newly synthesized prostaglandins from cells. These different functions likely operate in different compartments within the cell. OATP2A1 is reported to function at cytoplasmic vesicle/organelle membranes. As a regulator of the levels of physiologically active prostaglandins, OATP2A1 is implicated in diverse physiological and pathophysiological processes in many organs. Recently, whole exome analysis has revealed that recessive mutations in SLCO2A1 cause refractory diseases in humans, including primary hypertrophic osteoarthropathy (PHO) and chronic non-specific ulcers in small intestine (CNSU). Here, we review and summarize recent information on the molecular functions of OATP2A1 and on its physiological and pathological significance.

Similar content being viewed by others
Abbreviations
- AEC:
-
Alveolar epithelial cell
- CEAS:
-
Chronic enteropathy associated with SLCO2A1
- CMUSE:
-
Cryptogenic multifocal ulcerous stenosing enteritis
- CNSU:
-
Chronic non-specific ulcers in small intestine
- EP:
-
E prostanoid receptor
- HOA:
-
Hypertrophic osteoarthropathy
- OATP:
-
Organic anion transporting polypeptide(s)
- PHO:
-
Primary hypertrophic osteoarthropathy
- RMIC:
-
Renal medullary interstitial cell
- TMD:
-
Transmembrane domain
References
Kanai N, Lu R, Satriano JA, Bao Y, Wolkoff AW, Schuster VL. Identification and characterization of a prostaglandin transporter. Science. 1995;268:866–9.
Lu R, Kanai N, Bao Y, Schuster VL. Cloning, in vitro expression, and tissue distribution of a human prostaglandin transporter cDNA(hPGT). J Clin Invest. 1996;98:1142–9. https://doi.org/10.1172/JCI118897.
Lee SC, Levine L. Prostaglandin metabolism. II. Identification of two 15-hydroxyprostaglandin dehydrogenase types. J Biol Chem. 1975;250:548–52.
Anggard E, Larsson C. The sequence of the early steps in the metabolism of prostaglandin E1. Eur J Pharmacol. 1971;14:66–70.
Schuster VL. Molecular mechanisms of prostaglandin transport. Annu Rev Physiol. 1998;60:221–42. https://doi.org/10.1146/annurev.physiol.60.1.221.
Schuster VL. Prostaglandin transport. Prostaglandins Other Lipid Mediat. 2002;(68–69):633–47.
Nomura T, Lu R, Pucci ML, Schuster VL. The two-step model of prostaglandin signal termination: in vitro reconstitution with the prostaglandin transporter and prostaglandin 15 dehydrogenase. Mol Pharmacol. 2004;65:973–8. https://doi.org/10.1124/mol.65.4.973.
Nomura T, Chang HY, Lu R, Hankin J, Murphy RC, Schuster VL. Prostaglandin signaling in the renal collecting duct: release, reuptake, and oxidation in the same cell. J Biol Chem. 2005;280:28424–9. https://doi.org/10.1074/jbc.M408286200.
Zhang Z, Xia W, He J, Ke Y, Yue H, Wang C, et al. Exome sequencing identifies SLCO2A1 mutations as a cause of primary hypertrophic osteoarthropathy. Am J Hum Genet. 2012;90:125–32. https://doi.org/10.1016/j.ajhg.2011.11.019.
Seifert W, Kuhnisch J, Tuysuz B, Specker C, Brouwers A, Horn D. Mutations in the prostaglandin transporter encoding gene SLCO2A1 cause primary hypertrophic osteoarthropathy and isolated digital clubbing. Hum Mutat. 2012;33:660–4. https://doi.org/10.1002/humu.22042.
Umeno J, Hisamatsu T, Esaki M, Hirano A, Kubokura N, Asano K, et al. A hereditary enteropathy caused by mutations in the SLCO2A1 gene, encoding a prostaglandin transporter. PLoS Genet. 2015;11:e1005581. https://doi.org/10.1371/journal.pgen.1005581.
Chan BS, Satriano JA, Pucci M, Schuster VL. Mechanism of prostaglandin E2 transport across the plasma membrane of HeLa cells and Xenopus oocytes expressing the prostaglandin transporter “PGT”. J Biol Chem. 1998;273:6689–97. https://doi.org/10.1074/jbc.273.12.6689.
Roseman T, Yalkowsky S. Physicochemical properties of prostaglandin F2 alpha (tromethamine salt): solubility behavior, surface properties, and ionization constants. J Pharm Sci. 1973;62:1680–5.
Bito L, Baroody R. Impermeability of rabbit erythrocytes to prostaglandins. Am J Phys. 1975;229:1580–4.
Pucci ML, Bao Y, Chan B, Itoh S, Lu R, Copeland NG, et al. Cloning of mouse prostaglandin transporter PGT cDNA: species-specific substrate affinities. Am J Phys Renal Phys. 1999;277:R734–R41.
Banu SK, Arosh JA, Chapdelaine P, Fortier MA. Molecular cloning and spatio-temporal expression of the prostaglandin transporter: a basis for the action of prostaglandins in the bovine reproductive system. Proc Natl Acad Sci U S A. 2003;100:11747–52. https://doi.org/10.1073/pnas.1833330100.
Banu SK, Lee J, Satterfield MC, Spencer TE, Bazer FW, Arosh JA. Molecular cloning and characterization of prostaglandin (PG) transporter in ovine endometrium: role for multiple cell signaling pathways in transport of PGF2alpha. Endocrinology. 2008;149:219–31. https://doi.org/10.1210/en.2007-1087.
Gose T, Nakanishi T, Kamo S, Shimada H, Otake K, Tamai I. Prostaglandin transporter (OATP2A1/SLCO2A1) contributes to local disposition of eicosapentaenoic acid-derived PGE. Prostaglandins Other Lipid Mediat. 2016;122:10–7. https://doi.org/10.1016/j.prostaglandins.2015.12.003.
Itoh S, Lu R, Bao Y, Morrow JD, Roberts LJ, Schuster VL. Structural determinants of substrates for the prostaglandin transporter PGT. Mol Pharmacol. 1996;50:738–42.
Kraft ME, Glaeser H, Mandery K, Konig J, Auge D, Fromm MF, et al. The prostaglandin transporter OATP2A1 is expressed in human ocular tissues and transports the antiglaucoma prostanoid latanoprost. Invest Ophthalmol Vis Sci. 2010;51:2504–11. https://doi.org/10.1167/iovs.09-4290.
Kamo S, Nakanishi T, Aotani R, Nakamura Y, Gose T, Tamai I. Impact of FDA-approved drugs on the prostaglandin transporter OATP2A1/SLCO2A1. J Pharm Sci. 2017; https://doi.org/10.1016/j.xphs.2017.04.046.
Shirasaka Y, Shichiri M, Kasai T, Ohno Y, Nakanishi T, Hayashi K, et al. A role of prostaglandin transporter in regulating PGE2 release from human bronchial epithelial BEAS-2B cells in response to LPS. J Endocrinol. 2013;217:265–74. https://doi.org/10.1530/JOE-12-0339.
Chi Y, Khersonsky SM, Chang YT, Schuster VL. Identification of a new class of prostaglandin transporter inhibitors and characterization of their biological effects on prostaglandin E2 transport. J Pharmacol Exp Ther. 2006;316:1346–50. https://doi.org/10.1124/jpet.105.091975.
Chi Y, Min J, Jasmin JF, Lisanti MP, Chang YT, Schuster VL. Development of a high-affinity inhibitor of the prostaglandin transporter. J Pharmacol Exp Ther. 2011;339:633–41. https://doi.org/10.1124/jpet.111.181354.
Mandery K, Bujok K, Schmidt I, Wex T, Treiber G, Malfertheiner P, et al. Influence of cyclooxygenase inhibitors on the function of the prostaglandin transporter organic anion-transporting polypeptide 2A1 expressed in human gastroduodenal mucosa. J Pharmacol Exp Ther. 2010;332:345–51. https://doi.org/10.1124/jpet.109.154518.
Kulkarni PS, Srinivasan BD. Eicosapentaenoic acid metabolism in human and rabbit anterior uvea. Prostaglandins. 1986;31:1159–64.
Kulkarni PS, Srinivasan BD. Prostaglandins E3 and D3 lower intraocular pressure. Invest Ophthalmol Vis Sci. 1985;26:1178–82.
Suzuki Y OK, Inoue K, Yuasa H, editor. A rapid assay method for the assessment of the functionality of organic anion transporting polypeptide 2A1 by using a fluorescent substrate. The 135th Annual Meeting of the Pharmaceutical Society of Japan in Kobe; 2015 03/27.
Chan BS, Endo S, Kanai N, Schuster VL. Identification of lactate as a driving force for prostanoid transport by prostaglandin transporter PGT. Am J Phys Renal Phys. 2002;282:F1097–F102. https://doi.org/10.1152/ajprenal.00151.2001.
Chi Y, Pucci ML, Schuster VL. Dietary salt induces transcription of the prostaglandin transporter gene in renal collecting ducts. Am J Phys Renal Phys. 2008;295:F765–F71. https://doi.org/10.1152/ajprenal.00564.2007.
Nakanishi T, Hasegawa Y, Mimura R, Wakayama T, Uetoko Y, Komori H, et al. Prostaglandin transporter (PGT/SLCO2A1) protects the lung from bleomycin-induced fibrosis. PLoS One. 2015;10:e0123895. https://doi.org/10.1371/journal.pone.0123895.
Bao Y, Pucci ML, Chan BS, Lu R, Ito S, Schuster VL. Prostaglandin transporter PGT is expressed in cell types that synthesize and release prostanoids. Am J Phys Renal Phys. 2002;282:F1103–F10. https://doi.org/10.1152/ajprenal.00152.2001.
Pucci ML, Endo S, Nomura T, Lu R, Khine C, Chan BS, et al. Coordinate control of prostaglandin E2 synthesis and uptake by hyperosmolarity in renal medullary interstitial cells. Am J Phys Renal Phys. 2006;290:F641–F9. https://doi.org/10.1152/ajprenal.00426.2004.
Alzamil HA, Pawade J, Fortier MA, Bernal AL. Expression of the prostaglandin F synthase AKR1B1 and the prostaglandin transporter SLCO2A1 in human fetal membranes in relation to spontaneous term and preterm labor. Front Physiol. 2014;5:272. https://doi.org/10.3389/fphys.2014.00272.
Shimada H, Nakamura Y, Nakanishi T, Tamai I. OATP2A1/SLCO2A1-mediated prostaglandin E loading into intracellular acidic compartments of macrophages contributes to exocytotic secretion. Biochem Pharmacol. 2015;98:629–38. https://doi.org/10.1016/j.bcp.2015.10.009.
Bonney RJ, Wightman PD, Davies P, Sadowski SJ, Kuehl FA Jr, Humes JL. Regulation of prostaglandin synthesis and of the selective release of lysosomal hydrolases by mouse peritoneal macrophages. Biochem J. 1978;176:433–42.
Hsueh W, Kuhn C 3rd, Needleman P. Relationship of prostaglandin secretion by rabbit alveolar macrophages to phagocytosis and lysosomal enzyme release. Biochem J. 1979;184:345–54.
Kasai T, Nakanishi T, Ohno Y, Shimada H, Nakamura Y, Arakawa H, et al. Role of OATP2A1 in PGE2 secretion from human colorectal cancer cells via exocytosis in response to oxidative stress. Exp Cell Res. 2016;341:123–31. https://doi.org/10.1016/j.yexcr.2016.02.002.
Takeda S, Tanigawa T, Watanabe T, Tatsuwaki H, Nadatani Y, Otani K, et al. Reduction of prostaglandin transporter predicts poor prognosis associated with angiogenesis in gastric adenocarcinoma. J Gastroenterol Hepatol. 2016;31:376–83. https://doi.org/10.1111/jgh.13079.
Subra C, Grand D, Laulagnier K, Stella A, Lambeau G, Paillasse M, et al. Exosomes account for vesicle-mediated transcellular transport of activatable phospholipases and prostaglandins. J Lipid Res. 2010;51:2105–20. https://doi.org/10.1194/jlr.M003657.
Schuster VL, Itoh S, Andrews SW, Burk RM, Chen J, Kedzie KM, et al. Synthetic modification of prostaglandin f(2alpha) indicates different structural determinants for binding to the prostaglandin F receptor versus the prostaglandin transporter. Mol Pharmacol. 2000;58:1511–6.
Cui Y, König J, Leier I, Buchholz U, Keppler D. Hepatic uptake of bilirubin and its conjugates by the human organic anion transporter SLC21A6. J Biol Chem. 2001;276:9626–30. https://doi.org/10.1074/jbc.M004968200.
Jacquemin E, Hagenbuch B, Stieger B, Wolkoff AW, Meier PJ. Expression cloning of a rat liver Na(+)-independent organic anion transporter. Proc Natl Acad Sci U S A. 1994;91:133–7.
Chi Y, Jasmin JF, Seki Y, Lisanti MP, Charron MJ, Lefer DJ, et al. Inhibition of the prostaglandin transporter PGT lowers blood pressure in hypertensive rats and mice. PLoS One. 2015;10:e0131735. https://doi.org/10.1371/journal.pone.0131735.
Kashiwagi K, Kanai N, Tsuchida T, Suzuki M, Iizuka Y, Tanaka Y, et al. Comparison between isopropyl unoprostone and latanoprost by prostaglandin E(2)induction, affinity to prostaglandin transporter, and intraocular metabolism. Exp Eye Res. 2002;74:41–9. https://doi.org/10.1006/exer.2001.1104.
Ferreira SH, Vane JR. Prostaglandins: their disappearance from and release into the circulation. Nature. 1967;216:868–73. https://doi.org/10.1038/216868a0.
Holmes SW, Horton EW, Stewart MJ. Observations on the extraction of prostaglandins from blood. Life Sci. 1968;7:349–54.
Nakano J, Prancan AV. Metabolic degradation of prostaglandin E1 in the rat plasma and in rat brain, heart, lung, kidney and testicle homogenates. J Pharm Pharmacol. 1971;23:231–2.
Willman EA. The extraction of prostaglandin E 1 from human plasma. Life Sci. 1971;10:1181–91.
McDonald-Gibson WJ, McDonald-Gibson RG, Greaves MW. Metabolism of prostaglandin E 1 by human plasma. Biochem J. 1972;127:40P–1P.
Smith JB, Silver MJ, Ingerman CM, Kocsis JJ. Uptake and inactivation of a-type prostaglandins by human red cells. Prostaglandins. 1975;9:135–45.
Cozzini BO, Dawson CA. The role of the blood in metabolism of prostaglandin E1 in the cat lung. Prostaglandins. 1977;13:587–97.
Anderson MW, Eling TE. Prostaglandin removal and metabolism by isolated perfused rat lung. Prostaglandins. 1976;11:645–77.
Eling TE, Anderson MW. Studies on the biosynthesis, metabolism and transport of prostaglandins by the lung. Agents Actions. 1976;6:543–6.
Hagen AA, Gerber JN, Sweeley CC, White RP, Robertson JT. Levels and disappearance of prostaglandin F2alpha in cerebral spinal fluid: a clinical and experimental study. Stroke. 1977;8:672–5.
Dusting GJ, Moncada S, Vane JR. Recirculation of prostacyclin (PGI2) in the dog. Br J Pharmacol. 1978;64:315–20.
Wong PY, McGiff JC, Sun FF, Malik KU. Pulmonary metabolism of prostacyclin (PGI2) in the rabbit. Biochem Biophys Res Commun. 1978;83:731–8.
Topper JN, Cai J, Stavrakis G, Anderson KR, Woolf EA, Sampson BA, et al. Human prostaglandin transporter gene (hPGT) is regulated by fluid mechanical stimuli in cultured endothelial cells and expressed in vascular endothelium in vivo. Circulation. 1998;98:2396–403.
Pai JT, Ruoslahti E. Identification of endothelial genes up-regulated in vivo. Gene. 2005;347:21–33. https://doi.org/10.1016/j.gene.2004.12.034.
Tamai I, Nezu J-I, Uchino H, Sai Y, Oku A, Shimane M, et al. Molecular identification and characterization of novel members of the human organic anion transporter (OATP) family. Biochem Biophys Res Commun. 2000;273:251–60. https://doi.org/10.1006/bbrc.2000.2922.
Sekine T, Watanabe N, Hosoyamada M, Kanai Y, Endou H. Expression cloning and characterization of a novel multispecific organic anion transporter. J Biol Chem. 1997;272:18526–9. https://doi.org/10.1074/jbc.272.30.18526.
Nigam SK, Bush KT, Martovetsky G, Ahn SY, Liu HC, Richard E, et al. The organic anion transporter (OAT) family: a systems biology perspective. Physiol Rev. 2015;95:83–123. https://doi.org/10.1152/physrev.00025.2013.
Kimura H, Takeda M, Narikawa S, Enomoto A, Ichida K, Endou H. Human organic anion transporters and human organic cation transporters mediate renal transport of prostaglandins. J Pharmacol Exp Ther. 2002;301:293–8.
Shiraya K, Hirata T, Hatano R, Nagamori S, Wiriyasermkul P, Jutabha P, et al. A novel transporter of SLC22 family specifically transports prostaglandins and co-localizes with 15-hydroxyprostaglandin dehydrogenase in renal proximal tubules. J Biol Chem. 2010;285:22141–51. https://doi.org/10.1074/jbc.M109.084426.
Reid G, Wielinga P, Zelcer N, van der Heijden I, Kuil A, de Haas M, et al. The human multidrug resistance protein MRP4 functions as a prostaglandin efflux transporter and is inhibited by nonsteroidal antiinflammatory drugs. Proc Natl Acad Sci U S A. 2003;100:9244–9. https://doi.org/10.1073/pnas.1033060100.
Chang HY, Locker J, Lu R, Schuster VL. Failure of postnatal ductus arteriosus closure in prostaglandin transporter-deficient mice. Circulation. 2010;121:529–36. https://doi.org/10.1161/CIRCULATIONAHA.109.862946.
Hartney JM, Coggins KG, Tilley SL, Jania LA, Lovgren AK, Audoly LP, et al. Prostaglandin E2 protects lower airways against bronchoconstriction. Am J Phys Lung Cell Mol Phys. 2006;290:L105–L13. https://doi.org/10.1152/ajplung.00221.2005.
Olesen ET, Fenton RA. Is there a role for PGE2 in urinary concentration? J Am Soc Nephrol. 2013;24:169–78. https://doi.org/10.1681/ASN.2012020217.
Yang T, Singh I, Pham H, Sun D, Smart A, Schnermann JB, et al. Regulation of cyclooxygenase expression in the kidney by dietary salt intake. Am J Phys. 1998;274:F481–F9.
Jia Z, Zhang A, Zhang H, Dong Z, Yang T. Deletion of microsomal prostaglandin E synthase-1 increases sensitivity to salt loading and angiotensin II infusion. Circ Res. 2006;99:1243–51. https://doi.org/10.1161/01.RES.0000251306.40546.08.
Syeda MM, Jing X, Mirza RH, Yu H, Sellers RS, Chi Y. Prostaglandin transporter modulates wound healing in diabetes by regulating prostaglandin-induced angiogenesis. Am J Pathol. 2012;181:334–46. https://doi.org/10.1016/j.ajpath.2012.03.012.
Liu Z, Benard O, Syeda MM, Schuster VL, Chi Y. Inhibition of prostaglandin transporter (PGT) promotes perfusion and vascularization and accelerates wound healing in non-diabetic and diabetic rats. PLoS One. 2015;10:e0133615. https://doi.org/10.1371/journal.pone.0133615.
Owens JA, Falconer J, Robinson JS. Effect of restriction of placental growth on fetal and utero-placental metabolism. J Dev Physiol. 1987;9:225–38.
Seo H, Choi Y, Shim J, Yoo I, Ka H. Prostaglandin transporters ABCC4 and SLCO2A1 in the uterine endometrium and conceptus during pregnancy in pigs. Biol Reprod. 2014;90:100. https://doi.org/10.1095/biolreprod.113.114934.
Kowalewski MP, Kautz E, Hogger E, Hoffmann B, Boos A. Interplacental uterine expression of genes involved in prostaglandin synthesis during canine pregnancy and at induced prepartum luteolysis/abortion. Reprod Biol Endocrinol. 2014;12:46. https://doi.org/10.1186/1477-7827-12-46.
Kang J, Chapdelaine P, Parent J, Madore E, Laberge PY, Fortier MA. Expression of human prostaglandin transporter in the human endometrium across the menstrual cycle. J Clin Endocrinol Metab. 2005;90:2308–13. https://doi.org/10.1210/jc.2004-1482.
Fernandez-Martinez AB, Lucio-Cazana J. Intracellular EP2 prostanoid receptor promotes cancer-related phenotypes in PC3 cells. Cell Mol Life Sci. 2015;72:3355–73. https://doi.org/10.1007/s00018-015-1891-5.
Weinreb RN, Kashiwagi K, Kashiwagi F, Tsukahara S, Lindsey JD. Prostaglandins increase matrix metalloproteinase release from human ciliary smooth muscle cells. Invest Ophthalmol Vis Sci. 1997;38:2772–80.
Schuster VL, Lu R, Coca-Prados M. The prostaglandin transporter is widely expressed in ocular tissues. Surv Ophthalmol. 1997;41(Suppl 2):S41–S5.
Zhang P, Jiang B, Xie L, Huang W. PTGFR and SLCO2A1 gene polymorphisms determine intraocular pressure response to Latanoprost in Han Chinese patients with glaucoma. Curr Eye Res. 2016;41:1561–5. https://doi.org/10.3109/02713683.2016.1143013.
Yagami T, Koma H, Yamamoto Y. Pathophysiological roles of cyclooxygenases and prostaglandins in the central nervous system. Mol Neurobiol. 2016;53:4754–71. https://doi.org/10.1007/s12035-015-9355-3.
Wolfe LS, Coceani F. The role of prostaglandins in the central nervous system. Annu Rev Physiol. 1979;41:669–84. https://doi.org/10.1146/annurev.ph.41.030179.003321.
Choi K, Zhuang H, Crain B, Dore S. Expression and localization of prostaglandin transporter in Alzheimer disease brains and age-matched controls. J Neuroimmunol. 2008;195:81–7. https://doi.org/10.1016/j.jneuroim.2008.01.014.
Bito LZ, Davson H. Proceedings: carrier-mediated removal of prostaglandins from cerebrospinal fluid. J Physiol. 1974;236:39P–40P.
Tachikawa M, Ozeki G, Higuchi T, Akanuma S, Tsuji K, Hosoya K. Role of the blood-cerebrospinal fluid barrier transporter as a cerebral clearance system for prostaglandin E(2) produced in the brain. J Neurochem. 2012;123:750–60. https://doi.org/10.1111/jnc.12018.
Hosotani R, Inoue W, Takemiya T, Yamagata K, Kobayashi S, Matsumura K. Prostaglandin transporter in the rat brain: its localization and induction by lipopolysaccharide. Temperature. 2015;2:425–34. https://doi.org/10.1080/23328940.2015.1062953.
Akanuma S, Uchida Y, Ohtsuki S, Tachikawa M, Terasaki T, Hosoya K. Attenuation of prostaglandin E2 elimination across the mouse blood-brain barrier in lipopolysaccharide-induced inflammation and additive inhibitory effect of cefmetazole. Fluids Barriers CNS. 2011;8:24. https://doi.org/10.1186/2045-8118-8-24.
Uppal S, Diggle CP, Carr IM, Fishwick CW, Ahmed M, Ibrahim GH, et al. Mutations in 15-hydroxyprostaglandin dehydrogenase cause primary hypertrophic osteoarthropathy. Nat Genet. 2008;40:789–93. https://doi.org/10.1038/ng.153.
Yuksel-Konuk B, Sirmaci A, Ayten GE, Ozdemir M, Aslan I, Yilmaz-Turay U, et al. Homozygous mutations in the 15-hydroxyprostaglandin dehydrogenase gene in patients with primary hypertrophic osteoarthropathy. Rheumatol Int. 2009;30:39–43. https://doi.org/10.1007/s00296-009-0895-6.
Diggle CP, Carr IM, Zitt E, Wusik K, Hopkin RJ, Prada CE, et al. Common and recurrent HPGD mutations in Caucasian individuals with primary hypertrophic osteoarthropathy. Rheumatology (Oxford). 2010;49:1056–62. https://doi.org/10.1093/rheumatology/keq048.
Letts M, Pang E, Simons J. Prostaglandin-induced neonatal periostitis. J Pediatr Orthop. 1994;14:809–13.
Kozak KR, Milne GL, Morrow JD, Cuiffo BP. Hypertrophic osteoarthropathy pathogenesis: a case highlighting the potential role for cyclo-oxygenase-2-derived prostaglandin E2. Nat Clin Pract Rheumatol. 2006;2:452–6. https://doi.org/10.1038/ncprheum0252.
Diggle CP, Parry DA, Logan CV, Laissue P, Rivera C, Restrepo CM, et al. Prostaglandin transporter mutations cause pachydermoperiostosis with myelofibrosis. Hum Mutat. 2012;33:1175–81. https://doi.org/10.1002/humu.22111.
Sasaki T, Niizeki H, Shimizu A, Shiohama A, Hirakiyama A, Okuyama T, et al. Identification of mutations in the prostaglandin transporter gene SLCO2A1 and its phenotype-genotype correlation in Japanese patients with pachydermoperiostosis. J Dermatol Sci. 2012;68:36–44. https://doi.org/10.1016/j.jdermsci.2012.07.008.
Busch J, Frank V, Bachmann N, Otsuka A, Oji V, Metze D, et al. Mutations in the prostaglandin transporter SLCO2A1 cause primary hypertrophic osteoarthropathy with digital clubbing. J Investig Dermatol. 2012;132:2473–6. https://doi.org/10.1038/jid.2012.146.
Cheng R, Li M, Guo Y, Yao Y, Gao C, Yao Z. Three novel mutations in the SLCO2A1 gene in two Chinese families with primary hypertrophic osteoarthropathy. Eur J Dermatol. 2013;23:636–9. https://doi.org/10.1684/ejd.2013.2154.
Zhang Z, He JW, Fu WZ, Zhang CQ, Zhang ZL. Mutations in the SLCO2A1 gene and primary hypertrophic osteoarthropathy: a clinical and biochemical characterization. J Clin Endocrinol Metab. 2013;98:E923–E33. https://doi.org/10.1210/jc.2012-3568.
Zhang Z, He JW, Fu WZ, Zhang CQ, Zhang ZL. A novel mutation in the SLCO2A1 gene in a Chinese family with primary hypertrophic osteoarthropathy. Gene. 2013;521:191–4. https://doi.org/10.1016/j.gene.2013.03.047.
Niizeki H, Shiohama A, Sasaki T, Seki A, Kabashima K, Otsuka A, et al. The novel SLCO2A1 heterozygous missense mutation p.E427K and nonsense mutation p.R603* in a female patient with pachydermoperiostosis with an atypical phenotype. Br J Dermatol. 2014;170:1187–9. https://doi.org/10.1111/bjd.12790.
Ayoub N, Al-Khenaizan S, Sonbol H, Albreakan R, AlSufyani M, AlBalwi M. A novel homozygous mutation in the SLCO2A1 gene is associated with severe primary hypertrophic osteoarthropathy phenotype in a Saudi patient. Int J Dermatol. 2015;54:e233–5. https://doi.org/10.1111/ijd.12770.
Kim HJ, Koo KY, Shin DY, Kim DY, Lee JS, Lee MG. Complete form of pachydermoperiostosis with SLCO2A1 gene mutation in a Korean family. J Dermatol Sci. 2015;42:655–7. https://doi.org/10.1111/1346-8138.12856.
Lee S, Park SY, Kwon HJ, Lee CH, Kim OH, Rhee Y. Identification of the mutations in the prostaglandin transporter gene, SLCO2A1 and clinical characterization in Korean patients with pachydermoperiostosis. J Korean Med Sci. 2016;31:735–42. https://doi.org/10.3346/jkms.2016.31.5.735.
Mangupli R, Daly AF, Cuauro E, Camperos P, Krivoy J, Beckers A. Primary hypertrophic osteoarthropathy due to a novel SLCO2A1 mutation masquerading as acromegaly. Endocrinol Diabetes Metab Case Rep. 2017;2017 https://doi.org/10.1530/EDM-17-0013.
Niizeki H, Shiohama A, Sasaki T, Seki A, Kabashima K, Otsuka A, et al. The complete type of pachydermoperiostosis: a novel nonsense mutation p.E141* of the SLCO2A1 gene. J Dermatol Sci. 2014;75:193–5. https://doi.org/10.1016/j.jdermsci.2014.05.008.
Saadeh D, Kurban M, Ghosn S, Btadini W, Nemer G, Arayssi T, et al. Pachydermoperiostosis genetic screening in Lebanese families uncovers a novel SLCO2A1 mutation. J Eur Acad Dermatol Venereol. 2015;29:2489–90. https://doi.org/10.1111/jdv.12584.
Shigematsu YNH, Nozaki M, Sasaki R, Horikawa R, Seki A. A case of pachydermoperiostosis. Rinsho Hifuka. 2010;64:751–4.
Minakawa S, Kaneko T, Niizeki H, Mizukami H, Saito Y, Nigawara T, et al. Case of pachydermoperiostosis with solute carrier organic anion transporter family, member 2A1 (SLCO2A1) mutations. J Dermatol Sci. 2015;42:908–10. https://doi.org/10.1111/1346-8138.12974.
Li SS, He JW, Fu WZ, Liu YJ, Hu YQ, Zhang ZL. Clinical, biochemical, and genetic features of 41 Han Chinese families with primary hypertrophic osteoarthropathy, and their therapeutic response to Etoricoxib: results from a six-month prospective clinical intervention. J Bone Miner Res. 2017;32:1659–66. https://doi.org/10.1002/jbmr.3157.
Sinibaldi L, Harifi G, Bottillo I, Iannicelli M, El Hassani S, Brancati F, et al. A novel homozygous splice site mutation in the HPGD gene causes mild primary hypertrophic osteoarthropathy. Clin Exp Rheumatol. 2010;28:153–7.
Hatano R, Onoe K, Obara M, Matsubara M, Kanai Y, Muto S, et al. Sex hormones induce a gender-related difference in renal expression of a novel prostaglandin transporter, OAT-PG, influencing basal PGE2 concentration. Am J Phys Renal Phys. 2012;302:F342–F9. https://doi.org/10.1152/ajprenal.00366.2011.
Guda K, Fink SP, Milne GL, Molyneaux N, Ravi L, Lewis SM, et al. Inactivating mutation in the prostaglandin transporter gene, SLCO2A1, associated with familial digital clubbing, colon neoplasia, and NSAID resistance. Cancer Prev Res (Phila). 2014;(7):805–12. https://doi.org/10.1158/1940-6207.CAPR-14-0108.
Esaki M, Umeno J, Kitazono T, Matsumoto T. Clinicopathologic features of chronic nonspecific multiple ulcers of the small intestine. Clin J Gastroenterol. 2015;8:57–62. https://doi.org/10.1007/s12328-015-0559-x.
Perlemuter G, Guillevin L, Legman P, Weiss L, Couturier D, Chaussade S. Cryptogenetic multifocal ulcerous stenosing enteritis: an atypical type of vasculitis or a disease mimicking vasculitis. Gut. 2001;48:333–8.
Kohoutova D, Bartova J, Tacheci I, Rejchrt S, Repak R, Kopacova M, et al. Cryptogenic multifocal ulcerous stenosing enteritis: a review of the literature. Gastroenterol Res Pract. 2013;2013:918031. https://doi.org/10.1155/2013/918031.
Matsumoto T, Kubokura N, Matsui T, Iida M, Yao T. Chronic nonspecific multiple ulcer of the small intestine segregates in offspring from consanguinity. J Crohns Colitis. 2011;5:559–65. https://doi.org/10.1016/j.crohns.2011.05.008.
Hosoe N, Ohmiya N, Hirai F, Umeno J, Esaki M, Yamagami H, et al. Chronic enteropathy associated with SLCO2A1 gene (CEAS)—characterization of an enteric disorder to be considered in the differential diagnosis of Crohn’s disease. J Crohns Colitis. 2017; https://doi.org/10.1093/ecco-jcc/jjx068.
Adler DH, Cogan JD, Phillips JA 3rd, Schnetz-Boutaud N, Milne GL, Iverson T, et al. Inherited human cPLA(2alpha) deficiency is associated with impaired eicosanoid biosynthesis, small intestinal ulceration, and platelet dysfunction. J Clin Invest. 2008;118:2121–31. https://doi.org/10.1172/JCI30473.
Brooke MA, Longhurst HJ, Plagnol V, Kirkby NS, Mitchell JA, Ruschendorf F, et al. Cryptogenic multifocal ulcerating stenosing enteritis associated with homozygous deletion mutations in cytosolic phospholipase A2-alpha. Gut. 2014;63:96–104. https://doi.org/10.1136/gutjnl-2012-303581.
Backlund MG, Mann JR, Holla VR, Buchanan FG, Tai HH, Musiek ES, et al. 15-Hydroxyprostaglandin dehydrogenase is down-regulated in colorectal cancer. J Biol Chem. 2005;280:3217–23. https://doi.org/10.1074/jbc.M411221200.
Holla VR, Backlund MG, Yang P, Newman RA, DuBois RN. Regulation of prostaglandin transporters in colorectal neoplasia. Cancer Prev Res (Phila). 2008;1:93–9. https://doi.org/10.1158/1940-6207.CAPR-07-0009.
Kochel TJ, Goloubeva OG, Fulton AM. Upregulation of cyclooxygenase-2/prostaglandin E2 (COX-2/PGE2) pathway member multiple drug resistance-associated protein 4 (MRP4) and downregulation of prostaglandin transporter (PGT) and 15-prostaglandin dehydrogenase (15-PGDH) in triple-negative breast cancer. Breast Cancer (Auckl). 2016;(10):61–70. https://doi.org/10.4137/BCBCR.S38529.
Zeng Zhang, Jin-Wei He, Wen-Zhen Fu, Chang-Qing Zhang, Zhen-Lin Zhang, Two novel mutations in the SLCO2A1 gene in a Chinese patient with primary hypertrophic osteoarthropathy. Gene 2014;534(2):421-423. https://doi.org/10.1016/j.gene.2013.10.051.
Acknowledgments
This research was carried out with the support of a Grant-in-Aid for Scientific Research (KAKENHI, 15H04755) from the Japan Society for the Promotion of Science and the Smoking Research Foundation (Tokyo, Japan) to T. N. We also thank Dr. Kouichi Kawazu at Santen Pharmaceutical Co., Ltd. for technical asisitance for measuring intraocular pressure of mice.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict associated with this manuscript.
Additional information
Guest Editors: Wooin Lee and Takeo Nakanishi
Rights and permissions
About this article
Cite this article
Nakanishi, T., Tamai, I. Roles of Organic Anion Transporting Polypeptide 2A1 (OATP2A1/SLCO2A1) in Regulating the Pathophysiological Actions of Prostaglandins. AAPS J 20, 13 (2018). https://doi.org/10.1208/s12248-017-0163-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-017-0163-8