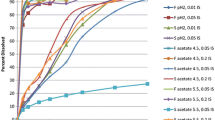
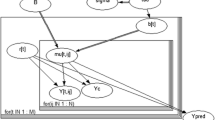
Abstract
The attention and interest in establishing in vivo/in vitro correlations (IVIVCs) is grounded in its tremendous utility as a prognostic tool. It can be used to support formulation optimization, predict in vivo drug exposure across a potential patient population, select a biologically relevant in vitro dissolution test condition, and support the use of in vitro dissolution data as a surrogate for in vivo bioequivalence trials. The pharmacological and statistical implications of this correlation are linked to the method by which the IVIVC was determined and to the assumptions and optimization approaches integrated into the estimation procedure. Using previously published data generated in normal healthy volunteers, an IVIVC for metoprolol was established using a mechanistic modeling approach. Within that framework, we explored the consequences of (1) our method of fitting a single Weibull function to the in vivo dissolution, (2) our selection of weighting scheme and optimization approaches, (3) the impact of applying a fixed versus fitted gastric emptying time, and 4) the importance of factoring population variability into our IVIVC estimation and profile reconvolution. We identified those factors found to be critical in terms of their influence on the accuracy of our predicted systemic metoprolol concentration-time profiles. We considered the strengths and weaknesses of our approach and discussed how the results of this study may impact efforts to generate IVIVCs with compounds presenting physicochemical characteristics different from that of metoprolol.





Similar content being viewed by others
References
Cardot JM, Beyssac E, Alric M. In vitro–in vivo correlation: importance of dissolution in IVIVC. Dissolution Technologies. 2007;15–19.
Rettig H, Mysicka J. IVIVC: methods and applications in modified-release product development. Dissolution Technologies. 2008;6–7.
Veng-Pedersen P, Gobburu JV, Meyer MC, Straughn AB. Carbamazepine level-A in vivo-in vitro correlation (IVIVC): a scaled convolution based predictive approach. Biopharm Drug Dispos. 2000;21(1):1–6.
Yu Z, Schwartz JB, Sugita ET, Foehl HC. Five modified numerical deconvolution methods for biopharmaceutics and pharmacokinetics studies. Biopharm Drug Dispos. 1996;17(6):521–40.
Cardot JM, Davit BM. In vitro-in vivo correlations: tricks and traps. AAPS J. 2012;14:491–9.
Lennernäs H, Aarons L, Augustijns P, Beato S, Bolger M, Box K, et al. Oral biopharmaceutics tools—time for a new initiative—an introduction to the IMI project OrBiTo. Eur J Pharm Sci. 2014;57:292–9.
Cutler DJ. Numerical deconvolution by least squares: use of polynomials to represent the input function. J Pharmacokinet Biopharm. 1978;6(3):243–63.
Cook JA. Development strategies for IVIVC in an industrial environment. Biopharm Drug Dispos. 2012;33:349–53.
Chan KK, Langenbucher F, Gibaldi M. Evaluation of in vivo drug release by numerical deconvolution using oral solution data as weighting function. J Pharm Sci. 1987;76(6):446–50.
Margolskee A, Darwich AS, Galetin A, Rostami-Hodjegan A, Aarons L. Deconvolution and IVIVC: exploring the role of rate-limiting conditions. AAPS J. 2015.
Sjögren E, Abrahamsson B, Augustijns P, Becker D, Bolger MB, Brewster M, et al. In vivo methods for drug absorption—comparative physiologies, model selection, correlations with in vitro methods (IVIVC), and applications for formulation/API/excipient characterization including food effects. Eur J Pharm Sci. 2014;57:99–151.
Nellore RV, Rekhi GS, Hussain AS, Tillman LG, Augsburger LL. Development of metoprolol tartrate extended-release matrix tablet formulations for regulatory policy consideration. J Control Release. 1998;50(1–3):247–56.
Eddington ND, Marroum P, Uppoor R, Hussain A, Augsburger L. Development and internal validation of an in vitro-in vivo correlation for a hydrophilic metoprolol tartrate extended release tablet formulation. Pharm Res. 1998;15:466–73. Erratum in: Pharm Res 1998;15(8):1320.
Mahayni H, Rekhi GS, Uppoor RS, Marroum P, Hussain AS, Augsburger LL, et al. Evaluation of “external” predictability of an in vitro-in vivo correlation for an extended-release formulation containing metoprolol tartrate. J Pharm Sci. 2000;89(10):1354–61.
Hildebrand M, Seifert W, Reichenberger A. Determination of dextromethorphan metabolizer phenotype in healthy volunteers. Eur J Clin Pharmacol. 1989;36(3):315–8.
Mistry B, Leslie J, Eddington NE. A sensitive assay of metoprolol and its major metabolite alpha-hydroxy metoprolol in human plasma and determination of dextromethorphan and its metabolite dextrorphan in urine with high performance liquid chromatography and fluorometric detection. J Pharm Biomed Anal. 1998;16(6):1041–9.
Jones H, Rowland-Yeo K. Basic concepts in physiologically based pharmacokinetic modeling in drug discovery and development. CPT Pharmacometrics Syst Pharmacol. 2013;2, e63.
Rodgers T, Rowland M. Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J Pharm Sci. 2006;95(6):1238–57. Erratum in: J Pharm Sci. 2007;96(11):3153–4.
Regårdh CG, Jordö L, Ervik M, Lundborg P, Olsson R, Rönn O. Pharmacokinetics of metoprolol in patients with hepatic cirrhosis. Clin Pharmacokinet. 1981;6(5):375–88.
Schaaf LJ, Campbell SC, Mayersohn MB, Vagedes T, Perrier DG. Influence of smoking and gender on the disposition kinetics of metoprolol. Eur J Clin Pharmacol. 1987;33(4):355–61.
Regårdh CG, Borg KO, Johansson R, Johnsson G, Palmer L. Pharmacokinetic studies on the selective beta1-receptor antagonist metoprolol in man. J Pharmacokinet Biopharm. 1974;2(4):347–64.
Lindahl A, Sandström R, Ungell AL, Abrahamsson B, Knutson TW, Knutson L, et al. Jejunal permeability and hepatic extraction of fluvastatin in humans. Clin Pharmacol Ther. 1996;60(5):493–503.
Peck CC, Sheiner LB, Nichols AI. The problem of choosing weights in nonlinear regression analysis of pharmacokinetic data. Drug Metab Rev. 1984;15(1–2):133–48.
Peck CC, Beal SL, Sheiner LB, Nichols AI. Extended least squares nonlinear regression: a possible solution to the “choice of weights” problem in analysis of individual pharmacokinetic data. J Pharmacokinet Biopharm. 1984;12(5):545–58.
Sheiner LB, Beal SL. Pharmacokinetic parameter estimates from several least squares procedures: superiority of extended least squares. J Pharmacokinet Biopharm. 1985;13(2):185–201.
Qiu, J, Martinez, M, Tiwari P. Evaluating in vivo in vitro correlations using a Bayesian approach. AAPS J (accepted for publication). 2016.
Madani S, Paine MF, Lewis L, Thummel KE, Shen DD. Comparison of CYP2D6 content and metoprolol oxidation between microsomes isolated from human livers and small intestines. Pharm Res. 1999;16(8):1199–205.
Parojcić J, Ibrić S, Djurić Z, Jovanović M, Corrigan OI. An investigation into the usefulness of generalized regression neural network analysis in the development of level A in vitro-in vivo correlation. Eur J Pharm Sci. 2007;30(3–4):264–72.
Dahan A, Lennernäs H, Amidon GL. The fraction dose absorbed, in humans, and high jejunal human permeability relationship. Mol Pharm. 2012;9(6):1847–51.
Neuhoff S, Ungell AL, Zamora I, Artursson P. pH-dependent bidirectional transport of weakly basic drugs across Caco-2 monolayers: implications for drug-drug interactions. Pharm Res. 2003;20(8):1141–8.
Sirisuth N, Augsburger LL, Eddington ND. Development and validation of a non-linear IVIVC model for a diltiazem extended release formulation. Biopharm Drug Dispos. 2002;23:1–8.
Mercuri A, Fares R, Bresciani M, Fotaki N. An in vitro-in vivo correlation study for nifedipine immediate release capsules administered with water, alcoholic and non-alcoholic beverages: Impact of in vitro dissolution media and hydrodynamics. Int J Pharm. 2016;499:330–42.
Mendell-Harary J, Dowell J, Bigora S, Piscitelli D, Butler J, Farrell C, et al. Nonlinear in vitro-in vivo correlations. Adv Exp Med Biol. 1997;423:199–206.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: Amin Rostami Hodjegan and Marilyn N. Martinez
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Fig. 1
(PDF 140 kb)
Supplemental Fig. 2
(PDF 198 kb)
Supplemental Fig. 3
(PDF 188 kb)
Supplemental Fig. 4
(PDF 175 kb)
Supplemental Fig. 5
(PDF 176 kb)
Supplemental Fig. 6
(PDF 193 kb)
Supplemental Fig. 7
(PDF 181 kb)
Supplemental Fig. 8
(PDF 232 kb)
Supplemental Fig. 9
(PDF 197 kb)
Supplemental Table 1
(PDF 181 kb)
Supplemental Table 2
(PDF 173 kb)
ESM 1
(PDF 362 kb)
Rights and permissions
About this article
Cite this article
Mistry, B., Patel, N., Jamei, M. et al. Examining the Use of a Mechanistic Model to Generate an In Vivo/In Vitro Correlation: Journey Through a Thought Process. AAPS J 18, 1144–1158 (2016). https://doi.org/10.1208/s12248-016-9930-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-016-9930-1