Abstract
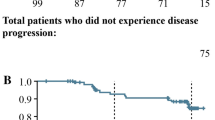
The objective of this work was to establish the quantitative relationship between Lanreotide Autogel® (LAN) on serum chromogranin A (CgA) and progression-free survival (PFS) in patients with nonfunctioning gastroenteropancreatic neuroendocrine tumors (GEP-NETs) through an integrated pharmacokinetic/pharmacodynamic (PK/PD) model. In CLARINET, a phase III, randomized, double-blind, placebo-controlled study, 204 patients received deep subcutaneous injections of LAN 120 mg (n = 101) or placebo (n = 103) every 4 weeks for 96 weeks. Data for 810 LAN and 1298 CgA serum samples (n = 632 placebo and n = 666 LAN) were used to develop a parametric time-to-event model to relate CgA levels and PFS (76 patients experienced disease progression: n = 49 placebo and n = 27 LAN). LAN serum profiles were described by a one-compartment disposition model. Absorption was characterized by two parallel pathways following first- and zero-order kinetics. As PFS data were considered informative dropouts, CgA and PFS responses were modeled jointly. The LAN-induced decrease in CgA levels was described by an inhibitory E MAX model. Patient age and target lesions at baseline were associated with an increment in baseline CgA. Weibull model distribution showed that decreases in CgA from baseline reduced the hazard of disease progression significantly (P < 0.001). Covariates of tumor location in the pancreas and tumor hepatic tumor load were associated with worse prognosis (P < 0.001). We established a semimechanistic PK/PD model to better understand the effect of LAN on a surrogate endpoint (serum CgA) and ultimately the clinical endpoint (PFS) in treatment-naive patients with nonfunctioning GEP-NETs.




Similar content being viewed by others
References
Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–72.
Ramage JK, Davies AH, Ardill J, Bax N, Caplin M, Grossman A, et al. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours. Gut. 2005;54 Suppl 4:iv1–16.
Arnold R, Wilke A, Rinke A, Mayer C, Kann PH, Klose K, et al. Plasma chromogranin A as marker for survival in patients with metastatic endocrine gastroenteropancreatic tumors. Clin Gastroenterol Hepatol. 2008;6:820–7.
Kaltsas GA, Besser GM, Grossman AB. The diagnosis and medical management of advanced neuroendocrine tumors. Endocr Rev. 2004;25:458–511.
Modlin IM, Lye KD, Kidd M. A 5‐decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–59.
Caplin ME, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedláčková E, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:224–33.
SOMATULINE DEPOT labeling revision 12/22/2014 reference ID: 3677425. http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/022074s010lbl.pdf.
Somatuline® autogel®. Summary of product characteristics (SmPC). Available from: https://www.medicines.org.uk/emc/medicine/25104.
Oberg K, Akerstrom G, Rindi G, Jelic S, ESMO Guidelines Working Group. Neuroendocrine gastroenteropancreatic tumours: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21 Suppl 5:v223–7.
Eriksson B, Oberg K, Stridsberg M. Tumor markers in neuroendocrine tumors. Digestion. 2000;62 Suppl 1:33–8.
Janson ET, Holmberg L, Stridsberg M, Eriksson B, Theodorsson E, Wilander E, et al. Carcinoid tumors: analysis of prognostic factors and survival in 301 patients from a referral center. Ann Oncol. 1997;8:685–90.
Granberg D, Wilander E, Stridsberg M, Granerus G, Skogseid B, Oberg K. Clinical symptoms, hormone profiles, treatment, and prognosis in patients with gastric carcinoids. Gut. 1998;43:223–8.
Kulke MH, Siu LL, Tepper JE, Fisher G, Jaffe D, Haller DG, et al. Future directions in the treatment of neuroendocrine tumors: consensus report of the National Cancer Institute neuroendocrine tumor clinical trials planning meeting. J Clin Oncol. 2011;29:934–43.
Italiano A. Prognostic or predictive? It’s time to get back to definitions! J Clin Oncol. 2011;29:4718. author reply 4718–9.
Sargent DJ, Conley BA, Allegra C, Collette L. Clinical trial designs for predictive marker validation in cancer treatment trials. J Clin Oncol. 2005;23:2020–7.
Pape UF, Berndt U, Muller-Nordhorn J, Bohmig M, Roll S, Koch M, et al. Prognostic factors of long-term outcome in gastroenteropancreatic neuroendocrine tumours. Endocr Relat Cancer. 2008;15:1083–97.
Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010;39:707–12.
Johanson V, Tisell LE, Olbe L, Wangberg B, Nilsson O, Ahlman H. Comparison of survival between malignant neuroendocrine tumours of midgut and pancreatic origin. Br J Cancer. 1999;80:1259–61.
Rinke A, Muller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID study group. J Clin Oncol. 2009;27:4656–63.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16.
Lindstrom ML, Bates DM. Nonlinear mixed effects models for repeated measures data. Biometrics. 1990;46:673–87.
Bauer R. NONMEM users guide introduction to NONMEM 7.2. 0. ICON Development Solutions Ellicott City, MD. 2011.
Buil-Bruna N, Garrido M, Dehez M, Manon A, Nguyen T, Trocóniz I. Population pharmacokinetic analysis of lanreotide depot/autogel in the treatment of neuroendocrine tumors: pooled analysis of four clinical trials. Clin Pharmacokinet. 2016. doi:10.1007/s40262-015-0329-4.
Box GEP, Cox DR. An analysis of transformations. J R Stat Soc Ser B Methodol. 1964;26:211–52.
Fox J, Weisberg S. An R companion to applied regression. 2nd ed. Thousand Oaks: Sage; 2011.
Post TM, Freijer JI, DeJongh J, Danhof M. Disease system analysis: basic disease progression models in degenerative disease. Pharm Res. 2005;22:1038–49.
Buil-Bruna N, López-Picazo J, Moreno-Jiménez M, Martín-Algarra S, Ribba B, Trocóniz IF. A population pharmacodynamic model for lactate dehydrogenase and neuron specific enolase to predict tumor progression in small cell lung cancer patients. AAPS J. 2014;16:609–19.
Sheiner LB, Stanski DR, Vozeh S, Miller RD, Ham J. Simultaneous modeling of pharmacokinetics and pharmacodynamics: application to d-tubocurarine. Clin Pharmacol Ther. 1979;25:358–71.
Dayneka NL, Garg V, Jusko WJ. Comparison of four basic models of indirect pharmacodynamic responses. J Pharmacokinet Biopharm. 1993;21:457–78.
Hu C, Sale M. A joint model for nonlinear longitudinal data with informative dropout. J Pharmacokinet Pharmacodyn. 2003;30:83–103.
Collett D. Modelling survival data in medical research. Boca Raton: CRC; 2003.
Lindbom L, Pihlgren P, Jonsson N. PsN-toolkit—a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Prog Biomed. 2005;79:241–57.
Nyberg J, Karlsson KE, Jönsson S, Simonsson USH, Karlsson MO, Hooker AC. Simulating large time-to-event trials in NONMEM. PAGE 23 (2014) Abstr 3166 [www.page-meeting.org/?abstract=3166].
Schoemaker RC, Van Gerven JM, Cohen AF. Estimating potency for the E max-model without attaining maximal effects. J Pharmacokinet Biopharm. 1998;26:581–93.
Bonate PL, Suttle B. Effect of censoring due to progressive disease on tumor size kinetic parameter estimates. AAPS J. 2013;15:832–9.
Panzuto F, Nasoni S, Falconi M, Corleto VD, Capurso G, Cassetta S, et al. Prognostic factors and survival in endocrine tumor patients: comparison between gastrointestinal and pancreatic localization. Endocr Relat Cancer. 2005;12:1083–92.
Kouvaraki MA, Ajani JA, Hoff P, Wolff R, Evans DB, Lozano R, et al. Fluorouracil, doxorubicin, and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. J Clin Oncol. 2004;22:4762–71.
Nikou G, Marinou K, Thomakos P, Papageorgiou D, Sanzanidis V, Nikolaou P, et al. Chromogranin a levels in diagnosis, treatment and follow-up of 42 patients with non-functioning pancreatic endocrine tumours. Pancreatology. 2008;8:510–9.
NCCN clinical practice guidelines in oncology/neuroendocrine tumors. Version 1.2015 [Internet]. [Cited accessed March 2015]. Available from: http://www.nccn.org/professionals/physician_gls/PDF/neuroendocrine.pdf.
Almufti R, Wilbaux M, Oza A, Henin E, Freyer G, Tod M, et al. A critical review of the analytical approaches for circulating tumor biomarker kinetics during treatment. Ann Oncol. 2014;25:41–56.
Keizer RJ, Schellens JH, Beijnen JH, Huitema AD. Pharmacodynamic biomarkers in model-based drug development in oncology. Curr Clin Pharmacol. 2011;6:30–40.
Kogan Y, Halevi-Tobias K, Elishmereni M, Vuk-Pavlović S, Agur Z. Reconsidering the paradigm of cancer immunotherapy by computationally aided real-time personalization. Cancer Res. 2012;72:2218–27.
You B, Harvey R, Henin E, Mitchell H, Golfier F, Savage P, et al. Early prediction of treatment resistance in low-risk gestational trophoblastic neoplasia using population kinetic modelling of hCG measurements. Br J Cancer. 2013;108:1810–6.
Kronik N, Kogan Y, Elishmereni M, Halevi-Tobias K, Vuk-Pavlović S, Agur Z. Predicting outcomes of prostate cancer immunotherapy by personalized mathematical models. PLoS One. 2010;5, e15482.
Hansson E, Ma G, Amantea M, French J, Milligan P, Friberg L, et al. PKPD modeling of predictors for adverse effects and overall survival in sunitinib-treated patients with GIST. CPT Pharmacometrics Syst Pharmacol. 2013;2, e85.
Hansson E, Amantea M, Westwood P, Milligan P, Houk B, French J, et al. PKPD modeling of VEGF, sVEGFR-2, sVEGFR-3, and sKIT as predictors of tumor dynamics and overall survival following sunitinib treatment in GIST. CPT Pharmacometrics Syst Pharmacol. 2013;2, e84.
Buil-Bruna N, Sahota T, Lopez-Picazo JM, Moreno-Jimenez M, Martin-Algarra S, Ribba B, et al. Early prediction of disease progression in small cell lung cancer: toward model-based personalized medicine in oncology. Cancer Res. 2015;75:2416–25.
Wilbaux M, Hénin E, Oza A, Colomban O, Pujade-Lauraine E, Freyer G, et al. Dynamic modeling in ovarian cancer: an original approach linking early changes in modeled longitudinal CA-125 kinetics and survival to help decisions in early drug development. Gynecol Oncol. 2014;133:460–6.
Bonate PL, Suttle AB. Modeling tumor growth kinetics after treatment with pazopanib or placebo in patients with renal cell carcinoma. Cancer Chemother Pharmacol. 2013;72:231–40.
Claret L, Girard P, Hoff PM, Van Cutsem E, Zuideveld KP, Jorga K, et al. Model-based prediction of phase III overall survival in colorectal cancer on the basis of phase II tumor dynamics. J Clin Oncol. 2009;27:4103–8.
Acknowledgments
The authors would like to thank Nicholas Brown, Senior Publications Officer, Ipsen Biopharm Ltd.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
This work was funded by Ipsen Pharma. Núria Buil-Bruna was supported by a predoctoral fellowship from Asociación de Amigos de la Universidad de Navarra. Marion Dehez, Amandine Manon, and Quyen Nguyen are employees of Ipsen which is the marketing authorization holder of Somatuline®, and Iñaki F. Trocóniz received research funding from Ipsen.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 16.7 kb)
Supplementary Fig. S1
Observed CgA levels distributions represented by histograms (upper panels) and Q-Q plots (lower panels) for nontransformed (natural scale), log-transformed and Box-Cox transformed CgA levels. (GIF 196 kb)
Supplementary Fig. S2
Relationship between observed baseline CgA levels and main covariates that showed statistical significance in the CgA model, including patient age, primary tumor location and number of lesions. Gray line shows the smooth curve fitted by the loess function. (GIF 266 kb)
Rights and permissions
About this article
Cite this article
Buil-Bruna, N., Dehez, M., Manon, A. et al. Establishing the Quantitative Relationship Between Lanreotide Autogel®, Chromogranin A, and Progression-Free Survival in Patients with Nonfunctioning Gastroenteropancreatic Neuroendocrine Tumors. AAPS J 18, 703–712 (2016). https://doi.org/10.1208/s12248-016-9884-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-016-9884-3