Abstract
Background and Objectives
Lanreotide Autogel® (lanreotide Depot in the USA) has demonstrated anti-tumor activity and control of the symptoms associated with hormone hypersecretion in patients with neuroendocrine tumors. The objectives of this study were to describe the pharmacokinetics of lanreotide Autogel® administered 4-weekly by deep subcutaneous injections of 60, 90, or 120 mg in patients with gastroenteropancreatic neuroendocrine tumors (GEP-NETs), to quantify the magnitude of inter-patient variability (IPV), and to identify those patient characteristics that impact on pharmacokinetics.
Methods
Analyses were based on pooled data from clinical trials. A total of 1541 serum concentrations from 290 patients were analyzed simultaneously by the population approach using NONMEM® version 7.2. Covariates evaluated included demographics, renal and hepatic function markers, and disease-related parameters.
Results
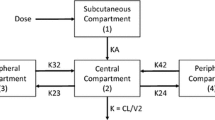
Serum profiles were described by a one-compartment disposition model in which the absorption process was characterized by two parallel pathways following first- and zero-order kinetics. The estimated apparent volume of distribution was 18.3 L. The estimated apparent total serum clearance for a typical 74 kg patient was 513 L/day, representing a substantial difference in clearance in this population of patients with respect to healthy volunteers that could not be explained by any of the covariates tested. Body weight was the only covariate to show a statistically significant effect on the pharmacokinetic profile, but due to the overlap between the pharmacokinetic profiles of patients with lower or higher body weights the effect of body weight on clearance was not considered clinically relevant. The IPV was low for clearance (27 %) and moderate to high for volume of distribution (150 %) and the absorption constant (61 %).
Conclusions
Using two mechanisms of absorption, the pharmacokinetics of lanreotide Autogel® were well-described in patients with GEP-NET. None of the patient characteristics tested were of clinical relevance to potential dose adjustment in clinical practice.






Similar content being viewed by others
References
Giustina A, Mazziotti G, Maffezzoni F, Amoroso V, Berruti A. Investigational drugs targeting somatostatin receptors for treatment of acromegaly and neuroendocrine tumors. Expert Opin Investig Drugs. 2014;23:1619–35.
FDA approved drug products. Highlights of prescribing information: Somatuline® depot (lanreotide) injection. http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/022074s011lbl.pdf. Accessed 15 Mar 2015.
Trocóniz IF, Cendrós JM, Peraire C, Ramis J, Garrido MJ, Boscani PF, et al. Population pharmacokinetic analysis of lanreotide Autogel in healthy subjects: evidence for injection interval of up to 2 months. Clin Pharmacokinet. 2009;48:51–62.
Barbanoj M, Antonijoan R, Morte A, Grinyó JM, Solà R, Vallès J, et al. Pharmacokinetics of the somatostatin analog lanreotide in patients with severe chronic renal insufficiency. Clin Pharmacol Ther. 1999;66:485–91.
Caplin ME, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedláčková E, CLARINET Investigators, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:224–33.
Vinik A, Wolin EM, Audry E, Gomez-Panzani EL, ELECT Study Group. ELECT: a phase 3 study of efficacy and safety of lanreotide autogel/depot (LAN) treatment for carcinoid syndrome in patients with neuroendocrine tumors (NETs) [abstract]. J Clin Oncol. 2014;32(Suppl. 3):268.
Martín-Richard M, Massutí B, Pineda E, Alonso V, Marmol M, Castellano D, TTD (Tumores del Tracto Digestivo) Study Group, et al. Antiproliferative effects of lanreotide autogel in patients with progressive, well-differentiated neuroendocrine tumours: a Spanish, multicentre, open-label, single arm phase II study. BMC Cancer. 2013;13:427.
Ruszniewski P, Ish-Shalom S, Wymenga M, O’Toole D, Arnold R, Tomassetti P, et al. Rapid and sustained relief from the symptoms of carcinoid syndrome: results from an open 6-month study of the 28-day prolonged-release formulation of lanreotide. Neuroendocrinology. 2004;80:244–51.
Beal S, Sheiner L, Boeckmann A. NONMEM users guide. Ellicott City: Icon Development Solutions; 1989–2006.
Lindbom L, Pihlgren P, Jonsson N. PsN-Toolkit—a collection of computer intensive statistical methods for nonlinear mixed effect modeling using NONMEM. Comput Methods Programs Biomed. 2005;79:241–57.
Ludden TM, Beal SL, Sheiner LB. Comparison of the akaike information criterion, the schwarz criterion and the F test as guides to model selection. J Pharmacokinet Pharmacodyn. 1994;22:431–45.
Wählby U, Thomson AH, Milligan PA, Karlsson MO. Models for time-varying covariates in population pharmacokinetic-pharmacodynamic analysis. Br J Clin Pharmacol. 2004;58:367–77.
Jonsson EN, Karlsson MO. Xpose—an S-PLUS based population pharmacokinetic/-pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed. 1999;58:51–64.
Karlsson MO, Savic RM. Diagnosing model diagnostics. Clin Pharmacol Ther. 2007;82:17–20.
Tornøe CW, Agersø H, Senderovitz T, Nielsen HA, Madsen H, Karlsson MO, et al. Population pharmacokinetic/pharmacodynamic (PK/PD) modelling of the hypothalamic-pituitary-gonadal axis following treatment with GnRH analogues. Br J Clin Pharmacol. 2007;63:648–64.
Romero E, Vélez de Mendizabal N, Cendrós JM, Peraire C, Bascompta E, Obach R, et al. Pharmacokinetic/pharmacodynamic model of the testosterone effects of triptorelin administered in sustained release formulations in patients with prostate cancer. J Pharmacol Exp Ther. 2012;342:788–98.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work has been founded by Ipsen Pharma.
Conflict of interest
Núria Buil-Bruna, María Jesús Garrido, and Iñaki F. Trocóniz have received research funding from Ipsen Pharma.
Marion Dehez, Amandine Manon, Thi Xuan Quyen Nguyen, and Edda L. Gomez-Panzani are employed by Ipsen Pharma.
Additional information
N. Buil-Bruna and M. J. Garrido contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
40262_2015_329_MOESM1_ESM.tiff
Supplementary material 1 (TIFF 833 kb) Supplementary Fig. S1. Goodness of fit plots corresponding to the selected population pharmacokinetic model. DV = observations; PRED = population model predictions; IPRED = individual model predictions; |IWRES| = absolute individual weighted residuals; CWRES = conditional weighted residuals; TAD = time after last administered dose. Lines in black show the perfect fit. Solid orange lines represent a smooth curve through the data
40262_2015_329_MOESM2_ESM.tif
Supplementary material 2 (TIFF 170 kb) Supplementary Fig. S2. Individual observed lanreotide serum concentration (points) and individual model predicted (solid lines) vs. time profiles for a sample of patients for each clinical trial (the first four patients in each trial dataset with at least two samples). Dashed lines show typical population predictions
40262_2015_329_MOESM3_ESM.tif
Supplementary material 3 (TIFF 2409 kb) Supplementary Fig. S3. Relationships between CWRES (conditional weighted residuals) and patient covariate values. Solid black lines represent the perfect fit (CWRES=0); solid blue lines show the smooth curve fitted by the Loess function. FCTN = 0 nonfunctioning, = 1 functioning NETs; TLOC, primary tumor location = 1 pancreas, = 2 foregut, = 3 midgut, = 4 hindgut, = 5 other, = 6 unknown
Rights and permissions
About this article
Cite this article
Buil-Bruna, N., Garrido, M.J., Dehez, M. et al. Population Pharmacokinetic Analysis of Lanreotide Autogel®/Depot in the Treatment of Neuroendocrine Tumors: Pooled Analysis of Four Clinical Trials. Clin Pharmacokinet 55, 461–473 (2016). https://doi.org/10.1007/s40262-015-0329-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-015-0329-4