Abstract
Previous studies suggested that an amorphous solid dispersion with a copolymer consisting of both hydrophobic and hydrophilic monomers could improve the dissolution profile of a poorly water-soluble drug compared to the crystalline form. Therefore, this study investigated the influence of the copolymer composition of polyvinylpyrrolidone/vinyl acetate (PVP/VA) on the non-sink in vitro dissolution behavior and in vivo performance of celecoxib (CCX) amorphous solid dispersions. The study showed that the hydrophilic monomer vinylpyrrolidone (VP) was responsible for the generation of CCX supersaturation whereas the hydrophobic monomer vinyl acetate (VA) was responsible for the stabilization of the supersaturated solution. For CCX, there was an optimal copolymer composition around 50–60% VP content where further replacement of VP monomers with VA monomers did not have any biopharmaceutical advantages. A linear relationship was found between the in vitro AUC0-4h and in vivo AUC0-24h for the CCX:PVP/VA systems, indicating that the non-sink in vitro dissolution method applied in this study was useful in predicting the in vivo performance. These results indicated that when formulating a poorly water-soluble drug as an amorphous solid dispersion using a copolymer, the copolymer composition has a significant influence on the dissolution profile and in vivo performance. Thus, the dissolution profile of a drug can theoretically be tailored by changing the monomer ratio of a copolymer with respect to the required in vivo plasma-concentration profile. As this ratio is likely to be drug dependent, determining the optimal ratio between the hydrophilic (dissolution enhancing) and hydrophobic (crystallization inhibiting) monomers for a given drug is imperative.
Similar content being viewed by others
INTRODUCTION
Development of formulation strategies to overcome the limited bioavailability associated with the increasing number of poorly water-soluble drug candidates is one of the most important challenges facing the pharmaceutical industry (1,2). It is well known that the utilization of the amorphous form may increase the apparent solubility and dissolution rate of a drug as a consequence of increased internal free energy. However, due to the high internal free energy and molecular mobility, amorphous materials also tend to crystallize (3–5). In order to circumvent crystallization and subsequent loss of the dissolution advantage, the amorphous drug can be dispersed in an amorphous polymer; a formulation strategy commonly referred to as an amorphous solid dispersion (6,7). Besides stabilizing the drug against crystallization in the solid state, the polymer can also further improve the dissolution profile through inhibition of the crystallization from the supersaturated solution generated upon dissolution (8,9).
The development of a successful amorphous solid dispersion is based on its dissolution performance after oral administration. Generally, the goal is to obtain a so-called spring and parachute dissolution profile (10). This means that the drug should dissolve rapidly to reach a supersaturated concentration (spring effect) followed by crystallization inhibition in order to maintain the supersaturation long enough for the drug to be absorbed (parachute effect) (11,12). Even though the underlying processes that govern the dissolution of amorphous solid dispersions are still not fully understood, the stabilization against crystallization is thought to be attributed to specific intermolecular interactions between the drug and polymer (13,14). Under non-sink dissolution conditions, both the dissolution rates and supersaturation levels obtained from amorphous solid dispersions have been reported to be higher with water-soluble (hydrophilic) carriers compared to systems with water-insoluble (hydrophobic) carriers (15). This interlinks well with the fact that the two most commonly used carriers for marketed amorphous solid dispersion are the hydrophilic polymers hydroxypropyl methylcellulose (HPMC) and polyvinylpyrrolidone (PVP) (10,16).
Even though hydrophilic polymers can provide a good “spring effect,” the fast dissolution and high degree of supersaturation could also generate a driving force for crystallization (17). Furthermore, studies have suggested that hydrophobicity is an important polymer property with respect to crystallization inhibition (15,18). This means that the fastest dissolving system may not necessarily show the best in vivo performance, and, hence, that the choice of polymer(s) will have a great effect on the dissolution profile and bioavailability of the amorphous solid dispersion (8,17). Consequently, in order to limit the crystallization upon dissolution of the drug from an amorphous solid dispersion, a hydrophobic polymer could be used in combination with a hydrophilic polymer (a so-called third generation solid dispersion) (19). This opportunity was investigated by Xie and Taylor, who found that combining an effective crystallization inhibition polymer with a dissolution-enhancing polymer in an amorphous solid dispersion significantly improved the dissolution profile of the drug compared to any of the pure polymers (20).
Alternatively, a copolymer consisting of both hydrophobic and hydrophilic monomers could hypothetically improve both dissolution and delay crystallization of the supersaturated drug. In this case, finding the optimal ratio between the hydrophilic (dissolution enhancing) and hydrophobic (crystallization inhibiting) monomers would be the critical formulation parameter. Thus, it is possible that the dissolution profile of a drug from an amorphous solid dispersion can be tailored by changing the monomer ratio in a copolymer.
Even though this hypothesis seems straightforward, the influence of the copolymer composition on the performance of amorphous solid dispersions has, to the best of our knowledge, not yet been investigated systematically. In addition, despite the great potential of amorphous solid dispersions, the number of published in vivo studies is still limited (16). Therefore, the purpose of this study was to investigate the influence of copolymer composition on the non-sink in vitro dissolution behavior and in vivo performance of an amorphous solid dispersion using celecoxib (CCX) as model drug and polyvinylpyrrolidone-co-vinyl acetate (PVP/VA) as copolymer. This copolymer consists of the hydrophilic monomer vinylpyrrolidone (VP) and the hydrophobic monomer vinyl acetate (VA) and is available in different monomer ratios: PVP/VA 335, PVP/VA 535, PVP/VA 635, and PVP/VA 735 (with 30, 50, 60, and 70% VP, respectively). The performance of the amorphous solid dispersions with the different copolymer ratios will be compared with that of the pure homopolymers PVP and polyvinyl acetate (PVA).
METHODS AND MATERIALS
Materials
Celecoxib (CCX; M w = 381.37 g/mol) was purchased from Astatech Inc. (Bristol, PA, USA). Ibuprofen (M w = 206.29 g/mol) and Kollidon® 30 (PVP K30, M w = 44,000–54,000 g/mol) were purchased from BASF (Ludwigshafen, Germany). Methanol (≥99.9%), acetonitrile (≥99.9%), and ammonium dihydrogen phosphate were purchased from Sigma-Aldrich (St. Louis, MO, USA). Sodium chloride, disodium hydrogen phosphate, hydrogen chloride, monopotassium phosphate, phosphoric acid (85%), sodium dihydrogen phosphate monohydrate, and sodium hydroxide pellets were purchased from Merck (Darmstadt, Germany). SIF™ Powder instant biorelevant medium was purchased from Phares AG (Muttenz, Switzerland). Polyvinyl acetate (PVA; M w = 35,000–45,000 g/mol) was purchased from VWR Chemicals (Pool, England), and polyvinylpyrrolidone/vinyl acetate (PVP/VA) copolymer E-335 (PVP/VA 335, M w = 28,000 g/mol), PVP/VA copolymer E-535 (PVP/VA 535, M w = 36,700 g/mol), PVP/VA copolymer E-635 (PVP/VA 635, M w = 38,200 g/mol), and PVP/VA copolymer E-735 (PVP/VA 735, M w = 56,700 g/mol) were kindly supplied by Ashland Chemical Co. (Columbus, OH, USA). PVA was received as pellets and was therefore pulverized using a Tube Mill control (at 10,000 rpm) from IKA® (Staufen, Germany) and sieved through a 0.4-mm sieve. The PVP/VA copolymers were sourced as 50% (w/w) ethanol solutions and converted to the solid form by spray drying. The solutions were diluted with ethanol to 5% (w/w) and processed using the B-290 Mini Spray Dryer from Büchi (Flawil, Switzerland). Air was drawn through the open-loop system with an aspirator rate at 100% and a temperature of 140°C, and the pump speed was set to 30%, which resulted in an outlet temperature of approximately 80°C (21).
Sample Preparation
The amorphous solid dispersions and amorphous CCX were prepared by melt quenching. Drug and polymer were weighed (25:75 w/w) and mixed thoroughly using a mortar and pestle. The physical mixture was then spread evenly on aluminum foil covered with 50.8 mm PTFE (Teflon) extruded film tape mm from 3M (St. Paul, MN, USA) and placed in an APT.line™ model ED electrical furnace from Binder GmbH (Tuttlingen, Germany) at 168°C for 2 min. The mixture was removed from the furnace, cooled to room temperature, and pulverized using a mortar and pestle. This procedure was repeated once more for the amorphous solid dispersions. The resulting powders were sieved with a 0.4-mm sieve in order to screen out any large particles or agglomerates and stored in air-tight containers until use.
Solid State Characterization
X-ray powder diffraction (XRPD) measurements were performed on an X’Pert PRO MRD diffractometer (PANalytical, Almelo, the Netherlands) equipped with a TCU 100 temperature control unit and an X’Celerator detector using nickel-filtered CuKα radiation (λ = 1.5406 Å) at 45 kV and 40 mA. Samples were placed on zero background (0-BG) Si plates and measured over the angular range 3–40° 2θ at a scanning rate of 1.20° 2θ/min. Results were analyzed using X’Pert Data Viewer (version 1.2) software.
Thermal Analysis
Differential scanning calorimetry (DSC) thermograms were acquired using a DSC Q2000 calorimeter (TA Instruments Inc, New Castle, DE, USA). Sample powders (2–4 mg) were analyzed in Tzero Aluminium Hermetic pans with a perforated lid and scanned from −10 to 200°C at a heating rate of 5°C/min and purged with 50 mL/min pure nitrogen gas. Temperature and enthalpy of the DSC instrument were calibrated using indium as a standard. The melting temperature (T m, onset) and glass transition temperatures (T g, midpoint) were determined using the Universal Analysis 2000 (version 4.5A) software.
Preparation of Fasted State Simulated Intestinal Fluid
Fasted state simulated intestinal fluid (FaSSIF) was both utilized as dissolution medium for in vitro dissolution and as a suspension liquid for the in vivo studies. Phosphate buffer pH 6.5 was prepared in a 1000-mL volumetric flask, by dissolving 0.420 g sodium hydroxide, 3.954 g of sodium dihydrogen phosphate monohydrate, and 6.286 g sodium chloride in approximately 900 mL of demineralized water. The pH was then adjusted to 6.5 with 1 M sodium hydroxide or 1 M hydrogen chloride, and the buffer was diluted to 1000 mL with demineralized water. The FaSSIF was prepared by dissolving 2.240 g SIF™ Powder in 500 mL phosphate buffer pH 6.5 and stirred on a magnetic stirrer until the powder was dissolved and an opalescent solution was obtained. The solution was then diluted to 1000 mL with phosphate buffer pH 6.5, stirred on a magnetic stirrer for a minimum of 2 h, and degassed before use. The FaSSIF was used within 24 h as specified by the supplier. The water used was from a Millipore purification system (Billerica, MA, USA).
In Vitro Studies
The non-sink in vitro dissolution studies were conducted at 37 ± 0.5°C in 500 mL of FaSSIF in an USP type II apparatus (paddle method) operating at 100 rpm using a VK7010 dissolution tester integrated with a VK650A heater/circulator, both from VanKel Technology Group (Cary, NC, USA). Three hundred microliters of the freshly prepared and degassed FaSSIF was placed in each of the six round-bottomed vessels and heated to 37 ± 0.5°C. The remaining 200 mL FaSSIF was heated to 37 ± 0.5°C in a type B 8023 oven from Termaks (Bergen, Norway). In order to achieve non-sink conditions, formulation corresponding to 400 mg CCX (saturation solubility 34.1 μg/mL) was suspended in the preheated 200 mL FaSSIF and added to the vessel at 0 min. Aliquots of 2 mL were withdrawn at 1, 5, 10, 15, 20, 30, 45, 60, 90, 120, 180, 240, 360, and 1440 min and filtered using 0.22 μm PTFE hydrophilic membrane Q-Max syringe filters from Frisenette ApS (Knebel, Denmark). Of the filtered sample, 1 mL was diluted with 1 mL mobile phase in order to avoid crystallization and analyzed using high-performance liquid chromatography (HPLC, see below).
In Vivo Studies
Male Sprague-Dawley rats weighing in the range of 277–330 g at the day of the experiment were purchased from Charles River Laboratories (Sulzfeld, Germany). To avoid interactions with lipid components in food, all rats were fasted 16–20 h prior to the study and until 12 h after dosing. Water was available at all times. Immediately before oral dosing of 100 mg/kg body weight of CCX in a volume of 5 mL/kg, the formulations were suspended in FaSSIF using a magnetic stirrer. A total of 48 rats were randomly assigned to one of the following groups, each consisting of 6 animals: (i) crystalline CCX, (ii) amorphous CCX, (iii) amorphous solid dispersions (25:75 w/w) of CCX/PVA, (iv) CCX.PVP/VA 335, (v) CCX.PVP/VA 535, (vi) CCX.PVP/VA 635, CCX.PVP/VA 735, and (vii) CCX.PVP K30. Blood samples of 100–200 μL were collected at 0.5, 1, 2, 3, 4, 6, 8, 10, 12, and 24 h after administration by individual vein puncture and transferred to ethylenediaminetetraacetic acid (EDTA)-coated tubes to prevent coagulation. The blood samples were then centrifuged for 10 min at 3600×g with a Heraeus Multifuge 1 S-R from Thermo Scientific Inc. (Hanau, Germany). Plasma was subsequently transferred to labeled plastic tubes and stored at −80°C until analysis. After collection of the last sample, the animals were euthanized.
Analytical Method
A HPLC method was used for the quantification of CCX in the samples from all the in vitro and in vivo studies. The HPLC system consisted of an L-7400 UV-detector, T-6000 column oven, L-7200 auto sampler, L-7100 pump, and D-7000 interface from Merck-Hitachi LaChrom (Tokyo, Japan). A reverse phase X-Bridge C-18 column (4.6 × 150 mm, 3.5 μm) from Waters (Milford, MA, USA) was used for the separation, and the mobile phase consisted of a methanol: 20 mM ammonium phosphate buffer (65:35 v/v) adjusted to pH 2.35 ± 0.05 with phosphoric acid. A total of 25 μL of sample was eluted at a flow rate of 1 mL/min, and the effluent was detected at a wavelength of 230 nm after approximately 7 min. A calibration standard concentration (100 μg/mL) was injected in between every 12 samples. The concentration of CCX was then calculated using the mean value of the peak areas obtained from the injected calibration standard concentration. The standard curve was linear over the range 0–500 μg/mL.
The bioanalysis was conducted by adding 20 μL of 0.1 mg/mL ibuprofen dissolved in acetonitrile (internal standard) to 20 μL of plasma samples. This mixture was then vortex-mixed with 100 μL acetonitrile to precipitate the proteins and centrifuged for 10 min at 11,500 rpm in a Hereaeus Labofuge 400 (Thermo Scientific Inc., Germany). The supernatant was transferred to vials and analyzed by HPLC. Using this extraction procedure, the recovery of CCX was 95–98% for calibration standard concentrations with a lower quantification limit of 50 ng/mL CCX.
Statistical Analysis
The primary pharmacokinetic parameters, area under the plasma concentration-time curve (AUC), maximum plasma concentration (C max), and time to reach C max (t max), were obtained by non-compartmental analysis of the plasma data. An analysis of variance (ANOVA) followed by a Newman-Keuls post hoc test was performed for untransformed data in order to detect differences in the pharmacokinetic parameters C max and AUC0–24h using SigmaPlot 11.0 from Systat Software, Inc. (Chicago, IL, USA). The values of t max were analyzed using a Mann-Whitney rank sum test for the paired samples. A statistical p value < 0.05 was considered significant.
RESULTS AND DISCUSSION
Thermal Stability and Homogeneity of the Formulations
In recent years, melt extrusion and spray drying have become the most common techniques for the preparation of amorphous solid dispersions (19). As these techniques are complex and require relatively large amounts of drug and polymer, a simple melt-quenching procedure was applied in this study to imitate the melt extrusion process and to limit the time and compound consumption. Due to the relatively high temperature applied in melting techniques, it is possible that the drug or excipients may degrade during processing. However, as the thermal degradation of CCX, PVA, PVP/VA, and PVP starts well after the melt quenching temperature of 168°C (22–25), the thermal stability of the amorphous solid dispersions was ensured during processing (confirmed by HPLC).
After preparation, it was confirmed that all the formulations were completely amorphous, as evident from a diffuse halo with no Bragg peaks in the XRPD diffractogram, and homogenously mixed at a molecular level implied by only one glass transition temperature between that of the pure components in the DSC thermogram (data not shown). The drug/polymer weight ratio was fixed at 25:75 w/w to investigate the effect of the composition of the copolymer on the in vitro behavior and in vivo performance rather than the drug/polymer ratio.
Effect of Copolymer Composition on Drug Dissolution at Non-Sink Conditions
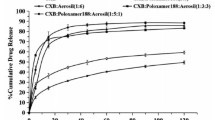
The dissolution profiles after non-sink in vitro dissolution of the different formulations in FaSSIF are shown in Fig. 1, and parameters descriptive for the dissolution profile are presented in Table I. As can be seen, the dissolution rates from amorphous CCX and CCX:PVP K30 were higher than for the other formulations. For the CCX:PVP/VA systems, the dissolution rate increased with increasing VP content. However, interestingly, the CCX:PVA system showed a lower dissolution rate than crystalline CCX. A previous study showed that the dissolution rates from sulfathiazole-PVP solid dispersions were highly depending on the molecular weight of the polymer (26). However, as the molecular weight of the polymers and copolymers used in this study was kept relatively constant (28,000–56,700 g/mol), this effect is thought to be due to the composition of the (co)polymers.
Dissolution profiles after non-sink in vitro dissolution of crystalline CCX (black circle), amorphous CCX (white circle), and amorphous solid dispersions of CCX in PVA (black triangle), PVP/VA 335 (white triangle), PVP/VA 535 (black square), PVP/VA 635 (white square), PVP/VA 735 (black diamond), and PVP K30 (white diamond) in FaSSIF at a dose corresponding to 0.8 mg/mL or a total of 400 mg of CCX from left, 0–24 h, and right, 0–4 h. Values represent mean CCX concentration ± SD (n = 3)
Furthermore, other studies indicated that drug release from amorphous solid dispersions is dependent on the wettability of the polymer matrix and thus viscosity and solubility in the medium (hydrophilicity) (26,27). The K value (viscosity in 1% w/v ethanol solution) for all the (co)polymers used in this study is around 30 (technical information, Ashland Chemical Co.). Assuming that the viscosity of the (co)polymers in FaSSIF is also equal, the effect of viscosity can be ignored, and hence, the increased dissolution rate may be isolated to the hydrophilicity of the (co)polymer. Therefore, the increasing dissolution rate is a function of copolymer composition as an increase in VP content will increase the overall hydrophilicity of the copolymer and thus the dissolution rate of the copolymer itself. These observations were all in accordance with a study by Sun and Lee, who suggested that for soluble (hydrophilic) polymers, the drug release mechanism is controlled by the dissolution of the drug, and for insoluble (hydrophobic) polymers, the drug release mechanism is controlled by the drug diffusion through the polymer matrix (15). However, the fastest dissolving system will not necessarily show the best in vivo performance as rapid generation of a supersaturated solution could generate a driving force for crystallization. Therefore, non-sink dissolution conditions are essential to enable a rational comparison of different polymers (17,28).
As can be seen from Table I, a direct correlation between the VP content and the maximum dissolution concentration was not observed. Even though the copolymers with the highest VP content offered the highest maximum concentration, there was no apparent correlation between the maximum drug concentration and copolymer composition (VP content). The maximum concentration for CCX:PVP/VA 635, CCX:PVP/VA 735, and CCX:PVP K30 was relatively similar (around 250 μg/mL), while CCX:PVP/VA 535 differed with a maximum concentration of almost 700 μg/mL (after 24 h)—more than twice as high as the formulation with the second highest concentration (CCX:PVP K30) and approximately 20 times higher than the equilibrium solubility of crystalline CCX (~35 μg/mL). The true cause for this phenomenon is still unknown, but it is likely that the hydrophobic VA monomer plays an important role.
This hypothesis is supported by observing the time to reach maximum concentration (Table I), where it is evident that CCX:PVA, CCX:PVP/VA 335, CCX:PVP/VA 535, and CCX:PVP/VA 635 did not crystallize during non-sink dissolution whereas amorphous CCX, CCX:PVP/VA 735, and CCX:PVP K30 crystallized. The amorphicity and crystallinity of the undissolved material/precipitate after dissolution was also confirmed by XRPD (data not shown). Consequently, the amorphous solid dispersions with (co)polymers containing a high content of VA monomers (40–100%) inhibited crystallization, which indicates that the hydrophobic VA monomer could be responsible for this.
According to the “spring and parachute effect,” the dissolution profile and thus overall performance of amorphous solid dispersions is essentially governed by two factors: generation and stabilization of a supersaturated solution (11). Consequently, in order to enable a ranking in relation to the overall in vitro performance of the different formulations, the area under the dissolution concentration-time curve (AUC0–24h) was calculated. Generally, it is recommended that the time frames used in in vitro studies are similar to those observed in biological systems when evaluating potential crystallization processes (28). Accordingly, as the combined transit time in the stomach and small intestine in fasted rats has been reported to be approximately 4 h (29) and another in vivo study on CCX suspensions also using male Sprague-Dawley rats found that the t max for CCX was around 4 h (30), the AUC0–4h was also calculated. Even though the two different in vitro AUCs deviate on the specific ranking, they both show that the formulation with (co)polymers containing a high VP content (CCX:PVP/VA 535, CCX:PVP/VA 635, CCX:PVP/VA 735, CCX:PVP/VA K30) performed better than the formulations containing no or low VP content (crystalline CCX, amorphous CCX, CCX:PVA, and CCX:PVP/VA 335). Furthermore, in both cases, the copolymers with a high VP content (CCX:PVP/VA 535, CCX:PVP/VA 635, and CCX:PVP/VA 735) also showed higher AUCs than the pure PVP homopolymer (CCX:PVP K30).
Based on the results from this in vitro study, the copolymer composition has significant influence on the dissolution profile of CCX. In summary, the VP monomer appeared to be responsible for the generation of supersaturation (dissolution enhancement) and the VA monomer for the stabilization of the supersaturated solution (crystallization inhibition). The optimal copolymer composition for CCX was around 50–70% VP content; further replacement of VP monomers with VA monomers or vice versa did not have any advantage in relation to the overall in vitro performance. As this optimal copolymer composition is most likely drug dependent, it must be anticipated that for a drug that has a strong tendency to crystallize upon dissolution, a higher content of the stabilizing VA monomer is required compared to a drug with a lower tendency to crystallize and vice versa. This means that, in theory, the release of drugs from amorphous solid dispersions can be controlled simply by modifying the composition of a given copolymer. In order to assess if the copolymer composition also has an influence on the bioavailability, an in vivo study was performed.
Effect of Copolymer Composition on In Vivo Performance in Rats
The mean plasma concentration-time profiles following oral administration of the different formulations are shown in Fig. 2. The calculated pharmacokinetic parameters, maximum plasma concentration (C max) of CCX, time to reach C max (t max), and area under the plasma concentration-time curve (AUC0–24h), are provided in Table II. As can be seen in Fig. 2, the amorphous solid dispersions with (co)polymers containing a high VP content (CCX:PVP/VA 535, CCX:PVP/VA 635, CCX:PVP/VA 735, and CCX:PVP K30) resulted in a faster absorption of CCX (concentration in the first blood sample) than the (co)polymers containing no or low VP content (crystalline CCX, amorphous CCX, CCX:PVA, and CCX:PVP/VA 335). This was also reflected in the C max, where the same four (co)polymers with high VP content had statistically significant higher values than the other four formulations. Furthermore, all formulations had a significantly higher C max than CCX:PVA, which was also in line with the observations from the in vitro dissolution study.
Plasma concentration-time profiles in rats after oral administrations of crystalline CCX (black circle), amorphous CCX (white circle), and amorphous solid dispersions of CCX in PVA (black triangle), PVP/VA 335 (white triangle), PVP/VA 535 (black square), PVP/VA 635 (white square), PVP/VA 735 (black diamond), and PVP K30 (white diamond) at a dose corresponding to 100 mg/kg body weight of CCX. Values represent mean CCX plasma concentration ± SEM (n = 6)
The data also revealed that the AUC0–24h of the different formulations was proportional to the C max, and thus, both parameters ranked the formulations CCX:PVP/VA 635 > CCX:PVP/VA 535 > CCX:PVP K30 > CCX:PVP/VA 735 > amorphous CCX > CCX:PVP/VA 335 > crystalline CCX > CCX:PVA. A statistical analysis of the data showed that the formulations with a (co)polymer containing a high VP content showed significantly higher C max and AUC0–24h than the formulations containing no or low VP content (except AUC0–24h for CCX:PVP/VA 735 vs amorphous CCX). In agreement with the statistical differences observed for C max, all formulations also had a significantly higher AUC0–24h than CCX:PVA. The reason for this is most likely that the drug is never released from the polymeric matrix because the polymer itself is never dissolved (as seen in vitro). This emphasizes that not all amorphous solid dispersions can increase the bioavailability of a poorly water-soluble drug, but in some cases, the bioavailability can actually be reduced.
For all the formulations, the t max was approximately 6 h, and no significant difference could be found. Hence, the short time to reach maximum concentration in vitro for amorphous CCX and CCX:PVP K30, due to crystallization during dissolution, was not reflected in vivo. A potential explanation for this could be that CCX crystallized in vivo before reaching the site of absorption, and thus, the majority of drug absorption did not commence until after CCX had crystallized. For formulations that have a potential to crystallize before the site of absorption, even small variations in transit times could have a major influence on the bioavailability (28), and therefore, it must be expected that these formulations provide the most variable bioavailability (AUC0–24h). This was in line with the observations in the present study on the four amorphous solid dispersions that provided a bioavailability (AUC0–24h) significantly larger than crystalline CCX. The bioavailability for the crystallizing formulations CCX:PVP K30 and CCX:PVP/VA 735 varied by 26 and 42%, respectively, whereas the bioavailability for the non-crystallizing formulations CCX:PVP/VA 535 and CCX:PVP 635 only varied by 10 and 15%, respectively.
As predicted from the non-sink in vitro dissolution tests, the in vivo study in rats confirmed that, except for CCX:PVA, the amorphous solid dispersions had a higher bioavailability than crystalline CCX. In accordance with the observations from the in vitro study, two of the copolymers PVP/VA 535 and PVP/VA 635 performed better than the other formulations, and therefore, it seems as if that there is an optimum copolymer composition around 50–60% VP content. Compared to the pure PVP homopolymer, inclusion of a VA monomer in a PVP/VA copolymer can increase the overall performance of an amorphous solid dispersion and lower the variability in the bioavailability significantly because of its ability to inhibit the crystallization of CCX.
The results of this study indicate that the copolymer composition (ratio between the hydrophilic monomer VP and hydrophobic monomer VA) has significant influence on the dissolution profile and in vivo performance of poorly water-soluble drugs. Furthermore, a recent study has also shown that the physical stability of the PVP/VA copolymer could be better than that of pure PVP due to an overall decrease of the hygroscopicity of the polymer (31). This means that replacing the hydrophilic VP repeat units with hydrophobic VA repeat units will not only significantly inhibit the crystallization upon dissolution of the amorphous solid dispersion, but it may also improve the physical stability of the formulation during storage (32,33). Consequently, knowledge about the optimum monomer ratio may be used advantageously in the future development of amorphous drug delivery systems as copolymers can theoretically be customized to “fit” any given drug.
In Vitro-In Vivo Correlation
The ultimate goal when performing in vitro studies is to establish a method that can predict the clinical performance of a formulation (in vivo performance) (28). Although the intraluminal behavior of amorphous solid dispersions still needs to be elucidated, the crystallization observed in vitro is also believed to occur in vivo. Therefore, in the case of supersaturating formulations, it is important that the in vitro dissolution behavior is evaluated in non-sink conditions to account for the effect of potential crystallization (17). Another crucial element is the comparison of relevant in vitro parameters with in vivo pharmacokinetic parameters. Of these pharmacokinetic parameters, the most important relating to the overall performance (bioavailability) of a formulation is the AUC. However, comparing the data from Tables I and II, no correlation between C max and maximum dissolution concentration or t max and time to reach maximum dissolution concentration could be established. Furthermore, no correlation was found between in vitro AUC0–24h and in vivo AUC0–24h.
Even though CCX is absorbed throughout the gastrointestinal tract, it is rational to assume that the majority of the dose is absorbed in the small intestine (34) as this constitutes more than 75% of the total length and 98% of the total surface volume of the gastrointestinal tract (35). The transit times in the stomach, small intestine, and large intestine of rats have been reported to be 15–30 min, 3–4 h, and 10–11 h, respectively (29). In comparison, the transit times in fasted humans have been reported to be 10–15 min (for liquids), 3–4 h, and 8–18 h, respectively (36). The combined transit time in the stomach and small intestine of both fasted humans and rats is thus approximately 4 h, and therefore, the in vitro AUC0–4h might be more predictive than the AUC0–24h. And in fact (CCX:PVA excluded), it was possible to establish an in vitro-in vivo correlation between in vitro AUC0–4h with in vivo AUC0–24h as illustrated in Fig. 3.
Relationships between in vitro AUC0–4h and in vivo AUC0–24h for crystalline CCX (black circle), amorphous CCX (white circle), and amorphous solid dispersions of CCX in PVA (black triangle), PVP/VA 335 (white triangle), PVP/VA 535 (black square), PVP/VA 635 (white square), PVP/VA 735 (black diamond), and PVP K30 (white diamond). Values for in vitro represent mean AUC0–4h ± SD (n = 3), and values for in vivo represent mean AUC0–24h ± SD (n = 6). The linear regression of the data points (CCX:PVA excluded) is plotted by a solid line (r 2 = 0.923)
The present study indicated that the in vitro non-sink dissolution method could be used to predict the in vivo performance of the formulations under investigation. However, it should be noted that the optimal in vitro time frame to predict in vivo performance could very well be drug dependent. Therefore, more investigations across compounds and (co)polymers need to be performed in order to confirm the predictive power of the non-sink dissolution method and the effect of copolymer composition on the in vivo performance in general.
CONCLUSION
Amorphous solid dispersions of CCX in PVA, PVP, and PVP/VA of different copolymer compositions were successfully prepared by a melt-quenching procedure. Except for CCX:PVA, all formulations showed an increased dissolution rate and apparent solubility compared to crystalline CCX. This improved dissolution behavior was also reflected in an increase in oral bioavailability in rats, which indicates that the absorption of CCX was solubility and dissolution limited. The formulations with (co)polymers containing a high VP content displayed superior in vitro and in vivo performance compared to those containing no VP or low VP content. The amorphous solid dispersion using the copolymers PVP/VA 535 and PVP/VA 635 performed better than the pure homopolymers PVA and PVP, and therefore, it seems that there is an optimum copolymer composition around 50–60% VP content. Of the two monomers contained in the copolymer, the hydrophilic VP monomer appeared responsible for the generation of supersaturation (dissolution enhancement) whereas the hydrophobic VA monomer was responsible for the stabilization of the supersaturated solution (crystallization inhibition). Finally, a correlation between in vitro AUC0–4h and in vivo AUC0–24h suggests that the in vitro non-sink dissolution method applied in this study could be used to predict in vivo performance.
The results of this study show that the implementation of the amorphous solid dispersion strategy holds great potential to increase the bioavailability of a poorly water-soluble drug if the right (co)polymeric carriers are chosen. The copolymer composition had significant influence on the dissolution profile and in vivo performance, which indicates that a copolymer consisting of a mix of hydrophobic and hydrophilic monomers, in theory, could improve both dissolution rate and delay crystallization of the supersaturated drug. Hence, it is possible that the dissolution profile can be tailored by changing the monomer ratio of a copolymer with respect to the required in vivo plasma-concentration profile. As this ratio is likely to be drug dependent, determining the optimal ratio between the hydrophilic (dissolution enhancing) and hydrophobic (crystallization inhibiting) monomers for a given drug is a critical formulation parameter when developing an amorphous solid dispersion with a copolymer such as PVP/VA as carrier.
References
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46(1-3):3–26.
Stegemann S, Leveiller F, Franchi D, de Jong H, Linden H. When poor solubility becomes an issue: from early stage to proof of concept. Eur J Pharm Sci. 2007;31(5):249–61.
Hancock BC, Zografi G. Characteristics and significance of the amorphous state in pharmaceutical systems. J Pharm Sci. 1997;86(1):1–12.
Murdande SB, Pikal MJ, Shanker RM, Bogner RH. Solubility advantage of amorphous pharmaceuticals: I. A thermodynamic analysis. J Pharm Sci. 2010;99(3):1254–64.
Laitinen R, Lobmann K, Strachan CJ, Grohganz H, Rades T. Emerging trends in the stabilization of amorphous drugs. Int J Pharm. 2013;453(1):65–79.
Vasconcelos T, Sarmento B, Costa P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov Today. 2007;12(23-24):1068–75.
Alonzo DE, Zhang GG, Zhou D, Gao Y, Taylor LS. Understanding the behavior of amorphous pharmaceutical systems during dissolution. Pharm Res. 2010;27(4):608–18.
Konno H, Handa T, Alonzo DE, Taylor LS. Effect of polymer type on the dissolution profile of amorphous solid dispersions containing felodipine. Eur J Pharm Biopharm. 2008;70(2):493–9.
Gupta P, Kakumanu VK, Bansal AK. Stability and solubility of celecoxib-PVP amorphous dispersions: a molecular perspective. Pharm Res. 2004;21(10):1762–9.
Huang Y, Wei-Guo D. Fundamental aspects of solid dispersion technology for poorly soluble drugs. Acta Pharm Sin B. 2014;4(1):18–25.
Guzman HR, Tawa M, Zhang Z, Ratanabanangkoon P, Shaw P, Gardner CR, et al. Combined use of crystalline salt forms and precipitation inhibitors to improve oral absorption of celecoxib from solid oral formulations. J Pharm Sci. 2007;96(10):2686–702.
Brouwers J, Brewster ME, Augustijns P. Supersaturating drug delivery systems: the answer to solubility-limited oral bioavailability? J Pharm Sci. 2009;98(8):2549–72.
Taylor LS, Zografi G. Spectroscopic characterization of interactions between PVP and indomethacin in amorphous molecular dispersions. Pharm Res. 1997;14(12):1691–8.
Miyazaki T, Yoshioka S, Aso Y, Kojima S. Ability of polyvinylpyrrolidone and polyacrylic acid to inhibit the crystallization of amorphous acetaminophen. J Pharm Sci. 2004;93(11):2710–7.
Sun DD, Lee PI. Probing the mechanisms of drug release from amorphous solid dispersions in medium-soluble and medium-insoluble carriers. J Control Release. 2015;211:85–93.
Shah N, Sandhu H, Choi D, Chokshi H, Malick AW. Amorphous solid dispersions: theory and practice. New York: Springer; 2014.
Augustijns P, Brewster ME. Supersaturating drug delivery systems: fast is not necessarily good enough. J Pharm Sci. 2012;101(1):7–9.
Ilevbare GA, Liu H, Edgar KJ, Taylor LS. Understanding polymer properties important for crystal growth inhibition-impact of chemically diverse polymers on solution crystal growth of ritonavir. Cryst Growth Des. 2012;12(6):3133–43.
Vo CLN, Park C, Lee BJ. Current trends and future perspectives of solid dispersions containing poorly water-soluble drugs. Eur J Pharm Biopharm. 2013;85(3, Part B):799–813.
Xie T, Taylor LS. Dissolution performance of high drug loading celecoxib amorphous solid dispersions formulated with polymer combinations. Pharm Res. 2016;33(1):1–12.
Knopp MM, Tajber L, Tian Y, Olesen NE, Jones DS, Kozyra A, et al. Comparative study of different methods for the prediction of drug-polymer solubility. Mol Pharm. 2015;12(9):3408–19.
Sovizi M. Thermal behavior of drugs: investigation on decomposition kinetic of naproxen and celecoxib. J Therm Anal Calorim. 2010;102(1):285–9.
Ballistreri A, Foti S, Montaudo G, Scamporrino E. Evolution of aromatic compounds in the thermal decomposition of vinyl polymers. J Polym Sci Polym Chem Ed. 1980;18(4):1147–53.
Zaccaron CM, Oliveira RVB, Guiotoku M, Pires ATN, Soldi V. Blends of hydroxypropyl methylcellulose and poly(1-vinylpyrrolidone-co-vinyl acetate): miscibility and thermal stability. Polym Degrad Stab. 2005;90(1):21–7.
Silva MF, Da Silva CA, Fogo FC, Pineda EAG, Hechenleitner AAW. Thermal and FTIR study of polyvinylpyrrolidone/lignin blends. J Therm Anal Calorim. 2005;79(2):367–70.
Simonelli AP, Mehta SC, Higuchi WI. Dissolution rates of high energy polyvinylpyrrolidone (PVP)-sulfathiazole coprecipitates. J Pharm Sci. 1969;58(5):538–49.
Hilton JE, Summers MP. The effect of wetting agents on the dissolution of indomethacin solid dispersion systems. Int J Pharm. 1986;31:157–64.
Newman A, Knipp G, Zografi G. Assessing the performance of amorphous solid dispersions. J Pharm Sci. 2012;101(4):1355–77.
Sjögren E, Abrahamsson B, Augustijns P, Becker D, Bolger MB, Brewster M, et al. In vivo methods for drug absorption—comparative physiologies, model selection, correlations with in vitro methods (IVIVC), and applications for formulation/API/excipient characterization including food effects. Eur J Pharm Sci. 2014;57:99–151.
Song WH, Yeom DW, Lee DH, Lee KM, Yoo HJ, Chae BR, et al. In situ intestinal permeability and in vivo oral bioavailability of celecoxib in supersaturating self-emulsifying drug delivery system. Arch Pharm Res. 2014;37(5):626–35.
Rask MB, Knopp MM, Olesen NE, Holm R, Rades T. Influence of PVP/VA copolymer composition on drug-polymer solubility. Eur J Pharm Sci 2015;Manuscript submitted for publication.
Taylor LS, Langkilde FW, Zografi G. Fourier transform Raman spectroscopic study of the interaction of water vapor with amorphous polymers. J Pharm Sci. 2001;90(7):888–901.
Prudic A, Kleetz T, Korf M, Ji Y, Sadowski G. Influence of copolymer composition on the phase behavior of solid dispersions. Mol Pharm. 2014;11(11):4189–98.
Paulson SK, Vaughn MB, Jessen SM, Lawal Y, Gresk CJ, Yan B, et al. Pharmacokinetics of celecoxib after oral administration in dogs and humans: effect of food and site of absorption. J Pharmacol Exp Ther. 2001;297(2):638–45.
Fadda HM, Sousa T, Carlsson AS, Abrahamsson B, Williams JG, Kumar D, et al. Drug solubility in luminal fluids from different regions of the small and large intestine of humans. Mol Pharm. 2010;7(5):1527–32.
Davis SS, Hardy JG, Fara JW. Transit of pharmaceutical dosage forms through the small intestine. Gut. 1986;27(8):886–92.
Acknowledgments
The staff in the animal facility at H. Lundbeck A/S is highly acknowledged for their skillful handling of the animals.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The procedure for the in vivo study was approved by the Animal Welfare Committee appointed by the Danish Ministry of Food, Agriculture, and Fisheries. All animal procedures were carried out in compliance with EC Directive 2010/63/EU, NIH Guide for the Care and Use of Laboratory Animals, and with the Danish laws regulating experiments on animals.
Rights and permissions
About this article
Cite this article
Knopp, M.M., Nguyen, J.H., Mu, H. et al. Influence of Copolymer Composition on In Vitro and In Vivo Performance of Celecoxib-PVP/VA Amorphous Solid Dispersions. AAPS J 18, 416–423 (2016). https://doi.org/10.1208/s12248-016-9865-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-016-9865-6