Abstract
Equivalence testing of aerodynamic particle size distribution (APSD) through multi-stage cascade impactors (CIs) is important for establishing bioequivalence of orally inhaled drug products. Recent work demonstrated that the median of the modified chi-square ratio statistic (MmCSRS) is a promising metric for APSD equivalence testing of test (T) and reference (R) products as it can be applied to a reduced number of CI sites that are more relevant for lung deposition. This metric is also less sensitive to the increased variability often observed for low-deposition sites. A method to establish critical values for the MmCSRS is described here. This method considers the variability of the R product by employing a reference variance scaling approach that allows definition of critical values as a function of the observed variability of the R product. A stepwise CI equivalence test is proposed that integrates the MmCSRS as a method for comparing the relative shapes of CI profiles and incorporates statistical tests for assessing equivalence of single actuation content and impactor sized mass. This stepwise CI equivalence test was applied to 55 published CI profile scenarios, which were classified as equivalent or inequivalent by members of the Product Quality Research Institute working group (PQRI WG). The results of the stepwise CI equivalence test using a 25% difference in MmCSRS as an acceptance criterion provided the best matching with those of the PQRI WG as decisions of both methods agreed in 75% of the 55 CI profile scenarios.





Similar content being viewed by others
Notes
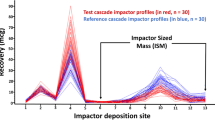
ISM is defined as the sum of the drug mass on all CI stages plus the filter, but excluding the initial stage because of its lack of a specified or well-defined upper cutoff diameter.
This was one of the main points, the PQRI working group criticized when evaluating the behavior of the original chi-square ratio method.
The distribution of the mCSRS when T and R products are identical is an approximate F distribution when certain conditions hold true (3).
This was not considered in previous work that was based on identifying general relationships between R variability and MmCSRS.
References
Lee SL, Adams WP, Li BV, Conner DP, Chowdhury BA, Yu LX. In vitro considerations to support bioequivalence of locally acting drugs in dry powder inhalers for lung diseases. AAPS J. 2009;11:414–23.
Evans C, Cipolla D, Chesworth T, Agurell E, Ahrens R, Conner D, et al. Equivalence considerations for orally inhaled products for local action-ISAM/IPAC-RS European workshop report. J Aerosol Med Pulm Drug Deliv. 2012;25:117–39.
Weber B, Hochhaus G, Adams W, Lionberger R, Li B, Tsong Y, et al. A stability analysis of a modified version of the chi-square ratio statistic: implications for equivalence testing of aerodynamic particle size distribution. AAPS J. 2013;15:1–9.
Weber B, Lee SL, Lionberger R, Li BV, Tsong Y, Hochhaus G. A sensitivity analysis of the modified chi-square ratio statistic for equivalence testing of aerodynamic particle size distribution. AAPS J. 2013;15:465–76.
Adams WP, Christopher D, Lee DS, Morgan B, Pan Z, Singh GJ, et al. Product Quality Research Institute evaluation of cascade impactor profiles of pharmaceutical aerosols, part 1: background for a statistical method. AAPS PharmSciTech. 2007;8:E32–7.
Christopher D, Adams W, Amann A, Bertha C, Byron PR, Doub W, et al. Product Quality Research Institute evaluation of cascade impactor profiles of pharmaceutical aerosols, part 3: final report on a statistical procedure for determining equivalence. AAPS PharmSciTech. 2007;8:65–74.
Christopher D, Adams WP, Lee DS, Morgan B, Pan Z, Singh GJ, et al. Product Quality Research Institute evaluation of cascade impactor profiles of pharmaceutical aerosols: part 2—evaluation of a method for determining equivalence. AAPS PharmSciTech. 2007;8:E39–48.
PQRI APSD Profile Comparisons Working Group. Realistic Scenarios 1–33 of 55. http://www.pqri.org/commworking/minutes/pdfs/dptc/psdpcwg/Addl/Realistic Scenarios 1–33 of 55.pdf. Accessed 08 June 2014
PQRI APSD Profile Comparisons Working Group. Realistic Scenarios 34–55 of 55. http://www.pqri.org/commworking/minutes/pdfs/dptc/psdpcwg/Addl/Realistic Scenarios 34–55 of 55.pdf. Accessed 08 June 2014
Hollander M, Wolfe DA. Nonparametric statistical methods. 2nd ed. New York: John Wiley & Sons; 1999. p. 178–86.
Labes D. Power and Sample size based on two one-sided t-tests (TOST) for (bio)equivalence studies. http://cran.r-project.org/web/packages/PowerTOST/PowerTOST.pdf. Accessed 08 June 2014
R Development Core Team. R: A language and environment for statistical computing. http://www.r-project.org/. Accessed 08 June 2014
Genz A, Bretz F, Miwa T, Mi X, Leisch F, Scheipl F, et al. Multivariate normal and t distributions. http://cran.r-project.org/web/packages/mvtnorm/mvtnorm.pdf. Accessed 08 June 2014
Efron B. Better bootstrap confidence intervals. JASA. 1987;82:171–85.
Weber B, Adams W, Lionberger R, Li B, Tsong Y, Hochhaus G, et al. Evaluation of statistical methods for determining equivalence of aerodynamic particle size distribution. Respir Drug Deliv. 2012;3:803–8.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: Lawrence Yu, Sau L. Lee, Guenther Hochhaus, Lana Lyapustina, Martin Oliver, and Craig Davies-Cutting
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Appendix
Appendix
Construction of 90% BCA Confidence Intervals for the MmCSRS
A sample size of 30 T and 30 R products will be assumed for simplicity. However, this method could be applied to any other sample size of T and R products. Moreover, this example assumes a bootstrapping sample size of 2000. For details and statistical background on the calculations, please refer to the original publication (14).
1. For both the T and R products obtain a bootstrapping sample with size equal to 30 by resampling with replacement.
2. Repeat (1) 2000 times.
3. Obtain the MmCSRS for each of the 2000 bootstrapping samples of 30 T and 30 R products.
4. For the distribution of 2000 MmCSRS that is obtained in (3), the Z L and Z U percentile of the 2000 MmCSRS are the lower and upper bounds of the BCA confidence interval, respectively. The computation of Z L and Z U is shown below.
where Φ is the cumulative distribution function of the standard normal distribution and \( {z}_{0.95} \) is the 95th percentile of the standard normal distribution
where
\( {\widehat{\theta}}_{-i} \) is the MmCSRS obtained for the original sample of 30 T and 30 R products by leaving a single T and R sample out (i.e., 900 MmCSRS based upon either 30 T and 29 R or 29 T and 30 R are obtained)
and
and
where I is an indicator function (i.e., equal to one if the condition is true and zero otherwise), \( {\widehat{\theta}}_b \) is the MmCSRS for bth bootstrapping sample, and \( \widehat{\theta} \) is the MmCSRS of the original sample.
Rights and permissions
About this article
Cite this article
Weber, B., Lee, S.L., Delvadia, R. et al. Application of the Modified Chi-Square Ratio Statistic in a Stepwise Procedure for Cascade Impactor Equivalence Testing. AAPS J 17, 370–379 (2015). https://doi.org/10.1208/s12248-014-9698-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-014-9698-0