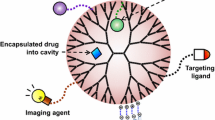
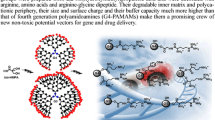
Abstract
Dendrimers are synthetic macromolecules composed of repetitive layers of branching units that emerge from a central core. They are characterized by a tunable size and precise number of peripheral groups which determine their physicochemical properties and function. Their high multivalency, functional surface, and globular architecture with diameters in the nanometer scale makes them ideal candidates for a wide range of applications. Gallic acid-triethylene glycol (GATG) dendrimers have attracted our attention as a promising platform in the biomedical field because of their high tunability and versatility. The presence of terminal azides in GATG dendrimers and poly(ethylene glycol) (PEG)-dendritic block copolymers allows their efficient functionalization with a variety of ligands of biomedical relevance including anionic and cationic groups, carbohydrates, peptides, or imaging agents. The resulting functionalized dendrimers have found application in drug and gene delivery, as antiviral agents and for the treatment of neurodegenerative diseases, in diagnosis and as tools to study multivalent carbohydrate recognition and dendrimer dynamics. Herein, we present an account on the preparation and recent applications of GATG dendrimers in these fields.










Similar content being viewed by others
Abbreviations
- AMCA:
-
Aminomethylcoumarin
- CA:
-
HIV capsid protein
- CTD:
-
C-Terminal domain
- Con A:
-
Concanavalin A
- CuAAC:
-
Cu(I)-catalyzed azide-alkyne cycloaddition
- DTPA:
-
Diethylenetriaminepentaacetate
- DOTA:
-
1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid
- DO3A:
-
Tri-tert-butyl 1,4,7,10-tetraazacyclododecane-1,4,7-triacetate
- EGFP:
-
Enhanced green fluorescent protein
- FDA:
-
Food and Drug Administration
- FITC:
-
Fluorescein isothiocyanate
- GATG:
-
Gallic acid-triethylene glycol
- G n :
-
Dendrimer generation, n denotes the generation number
- Glc:
-
Glucose
- HEK293T:
-
Human embryonic kidney cell line 293T
- HIV:
-
Human immunodeficiency virus
- HPMA:
-
N-(2-hydroxypropyl)methacrylamide
- HSV:
-
Herpes simplex virus
- ITC:
-
Isothermal titration calorimetry
- Man:
-
Mannose
- Mor:
-
Morpholine
- MRI:
-
Magnetic resonance imaging
- NOE:
-
Nuclear Overhauser effect
- PAMAM:
-
Polyamidoamine
- PEG:
-
Poly(ethylene glycol)
- PPI:
-
Polypropylene imine
- PIC:
-
Polyion complex
- RGD:
-
Arginylglycylaspartic acid
- SPR:
-
Surface plasmon resonance
References
Amaral SP, Fernandez-Villamarin M, Correa J, Riguera R, Fernandez-Megia E. Efficient multigram synthesis of the repeating unit of gallic acid-triethylene glycol dendrimers. Org Lett. 2011;13(17):4522–5.
Fréchet JMJ, Tomalia DA, editors. Dendrimers and other dendritic polymers. New York: Jonh Wiley & Sons; 2001
Tomalia DA. Birth of a new macromolecular architecture: dendrimers as quantized building blocks for nanoscale synthetic polymer chemistry. Prog Polym Sci. 2005;30(3–4):294–324.
Newkome GR, Moorefield CN, Vögtle F. Dendrimers and dendrons: concepts, syntheses, applications. Wiley-VCH: Weinheim; 2001.
Mintzer MA, Grinstaff MW. Biomedical applications of dendrimers: a tutorial. Chem Soc Rev. 2011;40(1):173–90.
Tekade RK, Kumar PV, Jain NK. Dendrimers in oncology: an expanding horizon. Chem Rev. 2009;109(1):49–87.
Medina SH, El-Sayed MEH. Dendrimers as carriers for delivery of chemotherapeutic agents. Chem Rev. 2009;109(7):3141–57.
Dufès C, Uchegbu IF, Schätzlein AG. Dendrimers in gene delivery. Adv Drug Delivery Rev. 2005;57(15):2177–202.
Rolland O, Turrin C-O, Caminade A-M, Majoral J-P. Dendrimers and nanomedicine: multivalency in action. New J Chem. 2009;33(9):1809–24.
Astruc D, Boisselier E, Ornelas C. Dendrimers designed for functions: from physical, photophysical, and supramolecular properties to applications in sensing, catalysis, molecular electronics, photonics, and nanomedicine. Chem Rev. 2010;110(4):1857–959.
Reek JNH, Arévalo S, van Heerbeek R, Kamer PCJ, van Leeuwen PWNM. Dendrimers in catalysis. In: Bruce CG, Helmut K, editors. Advances in catalysis: Academic Press; 2006. p. 71–151.
Rosen BM, Wilson CJ, Wilson DA, Peterca M, Imam MR, Percec V. Dendron-mediated self-assembly, disassembly, and self-organization of complex systems. Chem Rev. 2009;109(11):6275–540.
Buhleier E, Wehner W, Vogtle F. Synthesis. 1978;2:155–8.
Newkome GR, Yao Z, Baker GR, Gupta VK. Micelles. Part 1. Cascade molecules: a new approach to micelles. A [27]-arborol. J Org Chem. 1985;50(11):2003–4.
Tomalia DA, Baker H, Dewald J, Hall M, Kallos G, Martin S, et al. A new class of polymers: starburst-dendritic macromolecules. Polym J. 1985;17:117–32.
Esfand R, Tomalia DA. Poly(amidoamine) (PAMAM) dendrimers: from biomimicry to drug delivery and biomedical applications. Drug Discov Today. 2001;6(8):427–36.
de Brabander-vandenBerg EMM, Meijer EW. Poly(propylene imine) dendrimers: large-scale synthesis by hetereogeneously catalyzed hydrogenations. Angew Chem, Int Ed. 1993;32(9):1308–11.
Hawker CJ, Frechet JMJ. Preparation of polymers with controlled molecular architecture. A new convergent approach to dendritic macromolecules. J Am Chem Soc. 1990;112(21):7638–47.
Carnahan MA, Grinstaff MW. Synthesis of generational polyester dendrimers derived from glycerol and succinic or adipic acid. Macromolecules. 2005;39(2):609–16.
Ihre H, Padilla De Jesús OL, Fréchet JMJ. Fast and convenient divergent synthesis of aliphatic ester dendrimers by anhydride coupling. J Am Chem Soc. 2001;123(25):5908–17.
Majoral J-P, Caminade A-M. Dendrimers containing heteroatoms (Si, P, B, Ge, or Bi). Chem Rev. 1999;99(3):845–80.
Sashiwa H, Shigemasa Y, Roy R. Chemical modification of chitosan. 10.1 synthesis of dendronized chitosan–sialic acid hybrid using convergent grafting of preassembled dendrons built on gallic acid and tri(ethylene glycol) backbone. Macromolecules. 2001;34(12):3905–9.
Meunier SJ, Wu Q, Wang S-N, Roy R. Synthesis of hyperbranched glycodendrimers incorporating α-thiosialosides based on a gallic acid core. Can J Chem. 1997;75(11):1472–82.
Roy R, Park WKC, Wu Q, Wang S-N. Synthesis of hyper-branched dendritic lactosides. Tetrahedron Lett. 1995;36(25):4377–80.
Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. A stepwise huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew Chem, Int Ed. 2002;41(14):2596–9.
Tornøe CW, Christensen C, Meldal M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J Org Chem. 2002;67(9):3057–64.
Meldal M, Tornøe CW. Cu-catalyzed azide–alkyne cycloaddition. Chem Rev. 2008;108(8):2952–3015.
Lallana E, Sousa-Herves A, Fernandez-Trillo F, Riguera R, Fernandez-Megia E. Click chemistry for drug delivery nanosystems. Pharm Res. 2012;29(1):1–34.
Lallana E, Fernandez-Trillo F, Sousa-Herves A, Riguera R, Fernandez-Megia E. Click chemistry with polymers, dendrimers, and hydrogels for drug delivery. Pharm Res. 2012;29(4):902–21.
Wurm F, Frey H. Linear-dendritic block copolymers: the state of the art and exciting perspectives. Prog Polym Sci. 2011;36(1):1–52.
Gitsov I. Hybrid linear dendritic macromolecules: from synthesis to applications. J Polym Sci, Part A: Polym Chem. 2008;46(16):5295–314.
Sousa-Herves A, Riguera R, Fernandez-Megia E. PEG-dendritic block copolymers for biomedical applications. New J Chem. 2012;36:205–10.
Fernandez-Megia E, Correa J, Rodríguez-Meizoso I, Riguera R. A click approach to unprotected glycodendrimers. Macromolecules. 2006;39(6):2113–20.
Raviña M, de la Fuente M, Correa J, Sousa-Herves A, Pinto J, Fernandez-Megia E, et al. Core–shell dendriplexes with sterically induced stoichiometry for gene delivery. Macromolecules. 2010;43(17):6953–61.
Fernandez-Megia E, Correa J, Riguera R. Clickable PEG-dendritic block copolymers. Biomacromolecules. 2006;7(11):3104–11.
Gravert DJ, Janda KD. Organic synthesis on soluble polymer supports: liquid-phase methodologies. Chem Rev. 1997;97(2):489–510.
Jelínková M, Strohalm J, Etrych T, Ulbrich K, Říhová B. Starlike vs classic macromolecular prodrugs: two different antibody-targeted HPMA copolymers of doxorubicin studied in vitro and in vivo as potential anticancer drugs. Pharm Res. 2003;20(10):1558–64.
Khandare JJ, Jayant S, Singh A, Chandna P, Wang Y, Vorsa N, et al. Dendrimer versus linear conjugate: influence of polymeric architecture on the delivery and anticancer effect of paclitaxel. Bioconjugate Chem. 2006;17(6):1464–72.
Albertazzi L, Fernandez-Villamarin M, Riguera R, Fernandez-Megia E. Peripheral functionalization of dendrimers regulates internalization and intracellular trafficking in living cells. Bioconjugate Chem. 2012;23(5):1059–68.
Harada A, Kataoka K. Formation of polyion complex micelles in an aqueous milieu from a pair of oppositely-charged block copolymers with poly(ethylene glycol) segments. Macromolecules. 1995;28(15):5294–9.
Kabanov AV, Bronich TK, Kabanov VA, Yu K, Eisenberg A. Soluble stoichiometric complexes from poly(N-ethyl-4-vinylpyridinium) cations and poly(ethylene oxide)-block-polymethacrylate anions. Macromolecules. 1996;29(21):6797–802.
Lee Y, Kataoka K. Biosignal-sensitive polyion complex micelles for the delivery of biopharmaceuticals. Soft Matter. 2009;5:3810–7.
Miyata K, Christie RJ, Kataoka K. Polymeric micelles for nano-scale drug delivery. React Funct Polym. 2011;71(3):227–34.
Sousa-Herves A, Fernandez-Megia E, Riguera R. Synthesis and supramolecular assembly of clicked anionic dendritic polymers into polyion complex micelles. Chem Commun. 2008;27:3136–8.
Sousa-Herves A, Riguera R, Fernandez-Megia E. The pH-sensitive dendritic polymeric micelles as drug delivery systems. PCT Int Appl (2010) WO 2010018286 A1 20100218.
Mintzer MA, Simanek EE. Nonviral vectors for gene delivery. Chem Rev. 2009;109(2):259–302.
Liu X, Rocchi P, Peng L. Dendrimers as non-viral vectors for siRNA delivery. New J Chem. 2012;36(2):256–63.
Wood KC, Little SR, Langer R, Hammond PT. A family of hierarchically self-assembling linear-dendritic hybrid polymers for highly efficient targeted gene delivery. Angew Chem, Int Ed. 2005;44(41):6704–8.
Choi JS, Joo DK, Kim CH, Kim K, Park JS. Synthesis of a barbell-like triblock copolymer, poly(l-lysine) dendrimer-block-poly(ethylene glycol)-block-poly(l-lysine) dendrimer, and its self-assembly with plasmid DNA. J Am Chem Soc. 2000;122(3):474–80.
de la Fuente M, Ravina M, Sousa-Herves A, Correa J, Riguera R, Fernandez-Megia E, et al. Exploring the efficiency of gallic acid-based dendrimers and their block copolymers with PEG as gene carriers. Nanomedicine (Lond). 2012;7(11):1667–81.
Mishra S, Webster P, Davis ME. PEGylation significantly affects cellular uptake and intracellular trafficking of non-viral gene delivery particles. Eur J Cell Biol. 2004;83(3):97–111.
Chen CZ, Beck-Tan NC, Dhurjati P, van Dyk TK, LaRossa RA, Cooper SL. Quaternary ammonium functionalized poly(propylene imine) dendrimers as effective antimicrobials: structure–activity studies. Biomacromolecules. 2000;1(3):473–80.
Meyers SR, Juhn FS, Griset AP, Luman NR, Grinstaff MW. Anionic amphiphilic dendrimers as antibacterial agents. J Am Chem Soc. 2008;130(44):14444–5.
Ortega P, Copa-Patino JL, Munoz-Fernandez MA, Soliveri J, Gomez R, de la Mata FJ. Amine and ammonium functionalization of chloromethylsilane-ended dendrimers. Antimicrobial activity studies. Org Biomol Chem. 2008;6(18):3264–9.
Dernedde J, Rausch A, Weinhart M, Enders S, Tauber R, Licha K, et al. Dendritic polyglycerol sulfates as multivalent inhibitors of inflammation. Proc Natl Acad Sci U S A. 2010;107(46):19679–84.
Hayder M, Poupot M, Baron M, Nigon D, Turrin C-O, Caminade A-M, et al. A phosphorus-based dendrimer targets inflammation and osteoclastogenesis in experimental arthritis. Sci Transl Med. 2011;3(81):81–35.
Griffe L, Poupot M, Marchand P, Maraval A, Turrin C-O, Rolland O, et al. Multiplication of human natural killer cells by nanosized phosphonate-capped dendrimers. Angew Chem, Int Ed. 2007;46(14):2523–6.
Chonco L, Pion M, Vacas E, Rasines B, Maly M, Serramía MJ, et al. Carbosilane dendrimer nanotechnology outlines of the broad HIV blocker profile. J Control Release. 2012;161(3):949–58.
Jimenez JL, Pion M, Mata FJ, Gomez R, Munoz E, Leal M, et al. Dendrimers as topical microbicides with activity against HIV. New J Chem. 2012;36(2):299–309.
Blanzat M, Turrin C-O, Aubertin A-M, Couturier-Vidal C, Caminade A-M, Majoral J-P, et al. Dendritic catanionic assemblies: in vitro anti-HIV activity of phosphorus-containing dendrimers bearing Galβ1cer analogues. ChemBioChem. 2005;6(12):2207–13.
Rupp R, Rosentha SL, Stanberry LR. VivaGel™ (SPL7013 Gel): a candidate dendrimer—microbicide for the prevention of HIV and HSV infection. Int J Nanomed. 2007;4:561–6.
Supattapone S, Nguyen H-OB, Cohen FE, Prusiner SB, Scott MR. Elimination of prions by branched polyamines and implications for therapeutics. Proc Natl Acad Sci U S A. 1999;96(25):14529–34.
Klajnert B, Cangiotti M, Calici S, Majoral JP, Caminade AM, Cladera J, et al. EPR study of the interactions between dendrimers and peptides involved in Alzheimer’s and prion diseases. Macromol Biosci. 2007;7(8):1065–74.
Neira JL. The capsid protein of human immunodeficiency virus: designing inhibitors of capsid assembly. FEBS J. 2009;276(21):6110–7.
Doménech R, Abian O, Bocanegra R, Correa J, Sousa-Herves A, Riguera R, et al. Dendrimers as potential inhibitors of the dimerization of the capsid protein of HIV-1. Biomacromolecules. 2010;11(8):2069–78.
Xu L, Zhang H, Wu Y. Dendrimer advances for the central nervous system delivery of therapeutics. ACS Chem Neurosci. 2013;5(1):2–13.
Hindo SS, Mancino AM, Braymer JJ, Liu Y, Vivekanandan S, Ramamoorthy A, et al. Small molecule modulators of copper-induced Aβ aggregation. J Am Chem Soc. 2009;131(46):16663–5.
Yoo SI, Yang M, Brender JR, Subramanian V, Sun K, Joo NE, et al. Inhibition of amyloid peptide fibrillation by inorganic nanoparticles: functional similarities with proteins. Angew Chem, Int Ed. 2011;50(22):5110–5.
Klajnert B, Wasiak T, Ionov M, Fernandez-Villamarin M, Sousa-Herves A, Correa J, et al. Dendrimers reduce toxicity of Aβ 1–28 peptide during aggregation and accelerate fibril formation. Nanomedicine (New York, NY, U S). 2012;8:1372–8.
Pashkuleva I, Reis RL. Sugars: burden or biomaterials of the future? J Mater Chem. 2010;20(40):8803–18.
Fasting C, Schalley CA, Weber M, Seitz O, Hecht S, Koksch B, et al. Multivalency as a chemical organization and action principle. Angew Chem, Int Ed. 2012;51(42):10472–98.
Kiessling LL, Young T, Gruber TD, Mortell KH. Multivalency in protein–carbohydrate recognition. In: Fraser-Reid B, Tatsuta K, Thiem J, editors. Glycoscience. Heidelberg: Springer Berlin; 2008. p. 2483–523.
Lundquist JJ, Toone EJ. The cluster glycoside effect. Chem Rev. 2002;102(2):555–78.
Munoz EM, Correa J, Riguera R, Fernandez-Megia E. Real-time evaluation of binding mechanisms in multivalent interactions: a surface plasmon resonance kinetic approach. J Am Chem Soc. 2013;135(16):5966–9.
Munoz EM, Correa J, Fernandez-Megia E, Riguera R. Probing the relevance of lectin clustering for the reliable evaluation of multivalent carbohydrate recognition. J Am Chem Soc. 2009;131(49):17765–7.
Terreno E, Castelli DD, Viale A, Aime S. Challenges for molecular magnetic resonance imaging. Chem Rev. 2010;110(5):3019–42.
Geraldes CFGC, Laurent S. Classification and basic properties of contrast agents for magnetic resonance imaging. Contrast Media Mol Imaging. 2009;4(1):1–23.
Villaraza AJ, Bumb A, Brechbiel MW. Macromolecules, dendrimers, and nanomaterials in magnetic resonance imaging: the interplay between size, function, and pharmacokinetics. Chem Rev. 2010;110(5):2921–59.
Menjoge AR, Kannan RM, Tomalia DA. Dendrimer-based drug and imaging conjugates: design considerations for nanomedical applications. Drug Discov Today. 2010;15(5–6):171–85.
Louie A. Multimodality imaging probes: design and challenges. Chem Rev. 2010;110(5):3146–95.
Fernández-Trillo F, Pacheco-Torres J, Correa J, Ballesteros P, Lopez-Larrubia P, Cerdán S, et al. Dendritic MRI contrast agents: an efficient prelabeling approach based on CuAAC. Biomacromolecules. 2011;12(8):2902–7.
Dong Q, Hurst DR, Weinmann HJ, Chenevert TL, Londy FJ, Prince MR. Magnetic resonance angiography with gadomer-17: an animal study. Investig Radiol. 1998;33(9):699–708.
Matthias Ballauff CNL. Dendrimers in solution: insight from theory and simulation. Angew Chem, Int Ed. 2004;43(23):2998–3020.
Palmer AG. NMR characterization of the dynamics of biomacromolecules. Chem Rev. 2004;104(8):3623–40.
Pinto LF, Correa J, Martin-Pastor M, Riguera R, Fernandez-Megia E. The dynamics of dendrimers by NMR relaxation: interpretation pitfalls. J Am Chem Soc. 2013;135(5):1972–7.
Kowalewski J, Maeler L, Editors. Nuclear spin relaxation in liquids: theory, experiments, and applications: CRC Press; 2006.
Novoa-Carballal R, Säwén E, Fernandez-Megia E, Correa J, Riguera R, Widmalm G. The dynamics of GATG glycodendrimers by NMR diffusion and quantitative 13C relaxation. Phys Chem Chem Phys. 2010;12(25):6587–9.
Meltzer AD, Tirrell DA, Jones AA, Inglefield PT, Hedstrand DM, Tomalia DA. Chain dynamics in poly(amidoamine) dendrimers: a study of carbon-13 NMR relaxation parameters. Macromolecules. 1992;25(18):4541–8.
Hecht S, Fréchet JMJ. An alternative synthetic approach toward dendritic macromolecules: novel benzene-core dendrimers via alkyne cyclotrimerization. J Am Chem Soc. 1999;121(16):4084–5.
Kimata S-I, Jiang D-L, Aida T. Morphology-dependent luminescence properties of poly(benzyl ether) dendrimers. J Polym Sci, Part A: Polym Chem. 2003;41(22):3524–30.
Mourey TH, Turner SR, Rubinstein M, Frechet JMJ, Hawker CJ, Wooley KL. Unique behavior of dendritic macromolecules: intrinsic viscosity of polyether dendrimers. Macromolecules. 1992;25(9):2401–6.
Wooley KL, Klug CA, Tasaki K, Schaefer J. Shapes of dendrimers from rotational-echo double-resonance NMR. J Am Chem Soc. 1997;119(1):53–8.
Novoa-Carballal R, Fernandez-Megia E, Jimenez C, Riguera R. NMR methods for unravelling the spectra of complex mixtures. Nat Prod Rep. 2011;28(1):78–98.
Pinto LF, Riguera R, Fernandez-Megia E. Stepwise filtering of the internal layers of dendrimers by transverse-relaxation-edited NMR. J Am Chem Soc. 2013;135(31):11513–6.
Acknowledgments
The authors wish to acknowledge past and present lab members who have contributed to the development of dendrimers in our group. This work was financially supported by the Spanish Government (CTQ2009-10963, CTQ2012-34790, CTQ2009-14146-C02-02, CTQ2012-33436) and the Xunta de Galicia (10CSA209021PR and CN2011/037).
Conflict of Interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ana Sousa-Herves and Ramon Novoa-Carballal contributed equally to this work.
Rights and permissions
About this article
Cite this article
Sousa-Herves, A., Novoa-Carballal, R., Riguera, R. et al. GATG Dendrimers and PEGylated Block Copolymers: from Synthesis to Bioapplications. AAPS J 16, 948–961 (2014). https://doi.org/10.1208/s12248-014-9642-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-014-9642-3