Abstract
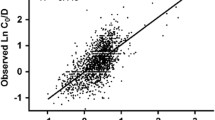
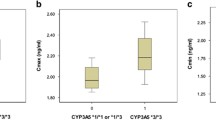
The SNP A6986G of the CYP3A5 gene (*3) results in a non-functional protein due to a splicing defect whereas the C3435T was associated with variable expression of the ABCB1 gene, due to protein instability. Part of the large interindividual variability in tacrolimus efficacy and toxicity can be accounted for by these genetic factors. Seventy-two individuals were examined for A6986G and C3435T polymorphism using a PCR-RFLP-based technique to estimate genotype and allele frequencies in the Jordanian population. The association of age, hematocrit, platelet count, CYP3A5, and ABCB1 polymorphisms with tacrolimus dose- and body-weight-normalized levels in the subset of 38 pediatric renal transplant patients was evaluated. A Markov model was used to evaluate the time-dependent probability of an adverse event occurrence by CYP3A5 phenotypes and ABCB1 genotypes. The time-dependent probability of adverse event was about double in CYP3A5 non-expressors compared to the expressors for the first 12 months of therapy. The CYP3A5 non-expressors had higher corresponding normalized tacrolimus levels compared to the expressors in the first 3 months. The correlation trend between probability of adverse events and normalized tacrolimus concentrations for the two CYP3A5 phenotypes persisted for the first 9 months of therapy. The differences among ABCB1 genotypes in terms of adverse events and normalized tacrolimus levels were only observed in the first 3 months of therapy. The information on CYP3A5 genotypes and tacrolimus dose requirement is important in designing effective programs toward management of tacrolimus side effects particularly for the initial dose when tacrolimus blood levels are not available for therapeutic drug monitoring.




Similar content being viewed by others
REFERENCES
Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2004;43(10):623–53.
Staatz CE, Goodman LK, Tett SE. Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: part II. Clin Pharmacokinet. 2010;49(4):207–21. doi:10.2165/11317550-000000000-000001.
Dai Y, Hebert MF, Isoherranen N, Davis CL, Marsh C, Shen DD, et al. Effect of CYP3A5 polymorphism on tacrolimus metabolic clearance in vitro. Drug Metab Dispos. 2006;34(5):836–47. doi:10.1124/dmd.105.008680.
Zheng H, Webber S, Zeevi A, Schuetz E, Zhang J, Bowman P, et al. Tacrolimus dosing in pediatric heart transplant patients is related to CYP3A5 and MDR1 gene polymorphisms. Am J Transplant. 2003;3(4):477–83.
Zheng H, Zeevi A, Schuetz E, Lamba J, McCurry K, Griffith BP, et al. Tacrolimus dosing in adult lung transplant patients is related to cytochrome P4503A5 gene polymorphism. J Clin Pharmacol. 2004;44(2):135–40. doi:10.1177/009127000326210844/2/135.
Thervet E, Anglicheau D, King B, Schlageter MH, Cassinat B, Beaune P, et al. Impact of cytochrome p450 3A5 genetic polymorphism on tacrolimus doses and concentration-to-dose ratio in renal transplant recipients. Transplantation. 2003;76(8):1233–5. doi:10.1097/01.TP.0000090753.99170.89.
Hoffmeyer S, Burk O, von Richter O, Arnold HP, Brockmoller J, Johne A, et al. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci U S A. 2000;97(7):3473–8. doi:10.1073/pnas.050585397050585397.
Fellay J, Marzolini C, Meaden ER, Back DJ, Buclin T, Chave JP, et al. Response to antiretroviral treatment in HIV-1-infected individuals with allelic variants of the multidrug resistance transporter 1: a pharmacogenetics study. Lancet. 2002;359(9300):30–6. doi:10.1016/S0140-6736(02)07276-8.
Staatz CE, Goodman LK, Tett SE. Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: part I. Clin Pharmacokinet. 2010;49(3):141–75. doi:10.2165/11317350-000000000-000001.
Hawwa AF, McKiernan PJ, Shields M, Millership JS, Collier PS, McElnay JC. Influence of ABCB1 polymorphisms and haplotypes on tacrolimus nephrotoxicity and dosage requirements in children with liver transplant. Br J Clin Pharmacol. 2009;68(3):413–21. doi:10.1111/j.1365-2125.2009.03461.x.
Herrlinger KR, Koc H, Winter S, Teml A, Stange EF, Fellermann K, et al. ABCB1 single-nucleotide polymorphisms determine tacrolimus response in patients with ulcerative colitis. Clin Pharmacol Ther. 2011;89(3):422–8. doi:10.1038/clpt.2010.348.
Choi JH, Lee YJ, Jang SB, Lee JE, Kim KH, Park K. Influence of the CYP3A5 and MDR1 genetic polymorphisms on the pharmacokinetics of tacrolimus in healthy Korean subjects. Br J Clin Pharmacol. 2007;64(2):185–91. doi:10.1111/j.1365-2125.2007.02874.x.
Mai I, Perloff ES, Bauer S, Goldammer M, Johne A, Filler G, et al. MDR1 haplotypes derived from exons 21 and 26 do not affect the steady-state pharmacokinetics of tacrolimus in renal transplant patients. Br J Clin Pharmacol. 2004;58(5):548–53. doi:10.1111/j.1365-2125.2004.02182.x.
Wang J, Zeevi A, McCurry K, Schuetz E, Zheng H, Iacono A, et al. Impact of ABCB1 (MDR1) haplotypes on tacrolimus dosing in adult lung transplant patients who are CYP3A5 *3/*3 non-expressors. Transpl Immunol. 2006;15(3):235–40. doi:10.1016/j.trim.2005.08.001.
MacPhee IA, Fredericks S, Tai T, Syrris P, Carter ND, Johnston A, et al. The influence of pharmacogenetics on the time to achieve target tacrolimus concentrations after kidney transplantation. Am J Transplant. 2004;4(6):914–9. doi:10.1111/j.1600-6143.2004.00435.xAJT435.
Thervet E, Loriot MA, Barbier S, Buchler M, Ficheux M, Choukroun G, et al. Optimization of initial tacrolimus dose using pharmacogenetic testing. Clin Pharmacol Ther. 2010;87(6):721–6. doi:10.1038/clpt.2010.17.
de Jonge H, de Loor H, Verbeke K, Vanrenterghem Y, Kuypers DR. In vivo CYP3A4 activity, CYP3A5 genotype, and hematocrit predict tacrolimus dose requirements and clearance in renal transplant patients. Clin Pharmacol Ther. 2012;92(3):366–75. doi:10.1038/clpt.2012.109.
Sy SK, Singh RP, Shilbayeh S, Zmeili R, Conrado D, Derendorf H. Influence of CYP3A5 6986A>G and ABCB1 3435C>T polymorphisms on adverse events associated with tacrolimus in Jordanian pediatric renal transplant patients. Clin Pharmacol Drug Dev. 2013;2(1):67–78. doi:10.1002/cpdd.22.
Shilbayeh S, Hazza I. Pediatric renal transplantation in the Jordanian population: the clinical outcome measures during long-term follow-up period. Pediatr Neonatol. 2012;53(1):24–33. doi:10.1016/j.pedneo.2011.11.006.
Bass RF. Stochastic processes. Cambridge: Cambridge University Press; 2011.
Lacroix BD, Lovern MR, Stockis A, Sargentini-Maier ML, Karlsson MO, Friberg LE. A pharmacodynamic Markov mixed-effects model for determining the effect of exposure to certolizumab pegol on the ACR20 score in patients with rheumatoid arthritis. Clin Pharmacol Ther. 2009;86(4):387–95. doi:10.1038/clpt.2009.136.
Ouellet D, Sutherland S, Wang T, Griffini P, Murthy V. First-time-in-human study with GSK1018921, a selective GlyT1 inhibitor: relationship between exposure and dizziness. Clin Pharmacol Ther. 2011;90(4):597–604. doi:10.1038/clpt.2011.154.
Bizzotto R, Zamuner S, Mezzalana E, De Nicolao G, Gomeni R, Hooker AC, et al. Multinomial logistic functions in Markov chain models of sleep architecture: internal and external validation and covariate analysis. AAPS J. 2011;13(3):445–63. doi:10.1208/s12248-011-9287-4.
Peng Z, Bao C, Zhao Y, Yi H, Xia L, Yu H, et al. Weighted Markov chains for forecasting and analysis in incidence of infectious diseases in Jiangsu Province, China. J Biomed Res. 2010;24(3):207–14. doi:10.1016/S1674-8301(10)60030-9.
Roy JN, Lajoie J, Zijenah LS, Barama A, Poirier C, Ward BJ, et al. CYP3A5 genetic polymorphisms in different ethnic populations. Drug Metab Dispos. 2005;33(7):884–7. doi:10.1124/dmd.105.003822.
Bhatnagar V, Garcia EP, O’Connor DT, Brophy VH, Alcaraz J, Richard E, et al. CYP3A4 and CYP3A5 polymorphisms and blood pressure response to amlodipine among African-American men and women with early hypertensive renal disease. Am J Nephrol. 2010;31(2):95–103. doi:10.1159/000258688.
Rao DN, Manjula G, Sailaja K, Surekha D, Raghunadharao D, Rajappa S, et al. Association of CYP3A5*3 polymorphism with development of acute leukemia. Indian J Hum Genet. 2011;17(3):175–8. doi:10.4103/0971-6866.92098.
Cho JH, Yoon YD, Park JY, Song EJ, Choi JY, Yoon SH, et al. Impact of cytochrome P450 3A and ATP-binding cassette subfamily B member 1 polymorphisms on tacrolimus dose-adjusted trough concentrations among Korean renal transplant recipients. Transplant Proc. 2012;44(1):109–14. doi:10.1016/j.transproceed.2011.11.004.
Zhao W, Elie V, Roussey G, Brochard K, Niaudet P, Leroy V, et al. Population pharmacokinetics and pharmacogenetics of tacrolimus in de novo pediatric kidney transplant recipients. Clin Pharmacol Ther. 2009;86(6):609–18. doi:10.1038/clpt.2009.210.
de Wildt SN, van Schaik RH, Soldin OP, Soldin SJ, Brojeni PY, van der Heiden IP, et al. The interactions of age, genetics, and disease severity on tacrolimus dosing requirements after pediatric kidney and liver transplantation. Eur J Clin Pharmacol. 2011;67(12):1231–41. doi:10.1007/s00228-011-1083-7.
Gijsen V, Mital S, van Schaik RH, Soldin OP, Soldin SJ, van der Heiden IP, et al. Age and CYP3A5 genotype affect tacrolimus dosing requirements after transplant in pediatric heart recipients. J Heart Lung Transplant. 2011;30(12):1352–9. doi:10.1016/j.healun.2011.08.001.
Kim JS, Aviles DH, Silverstein DM, Leblanc PL, Matti VV. Effect of age, ethnicity, and glucocorticoid use on tacrolimus pharmacokinetics in pediatric renal transplant patients. Pediatr Transplant. 2005;9(2):162–9. doi:10.1111/j.1399-3046.2005.00263.x.
Naesens M, Salvatierra O, Li L, Kambham N, Concepcion W, Sarwal M. Maturation of dose-corrected tacrolimus predose trough levels in pediatric kidney allograft recipients. Transplantation. 2008;85(8):1139–45. doi:10.1097/TP.0b013e31816b431a.
Sy SK, Johnston JA, Class CF, Roberts EA, Kalow W, Tang BK. Mulitvariate cluster analysis of a human hepatic cytochrome P450 database. Eur J Clin Pharmacol. 2002;58(8):559–62.
Sy SK, Ciaccia A, Li W, Roberts EA, Okey A, Kalow W, et al. Modeling of human hepatic CYP3A4 enzyme kinetics, protein, and mRNA indicates deviation from log-normal distribution in CYP3A4 gene expression. Eur J Clin Pharmacol. 2002;58(5):357–65. doi:10.1007/s00228-002-0487-9.
Montini G, Ujka F, Varagnolo C, Ghio L, Ginevri F, Murer L, et al. The pharmacokinetics and immunosuppressive response of tacrolimus in paediatric renal transplant recipients. Pediatr Nephrol. 2006;21(5):719–24. doi:10.1007/s00467-006-0014-9.
Yasuhara M, Hashida T, Toraguchi M, Hashimoto Y, Kimura M, Inui K, et al. Pharmacokinetics and pharmacodynamics of FK 506 in pediatric patients receiving living-related donor liver transplantations. Transplant Proc. 1995;27(1):1108–10.
Wallemacq PE, Furlan V, Moller A, Schafer A, Stadler P, Firdaous I, et al. Pharmacokinetics of tacrolimus (FK506) in paediatric liver transplant recipients. Eur J Drug Metab Pharmacokinet. 1998;23(3):367–70.
Hines RN. Ontogeny of human hepatic cytochromes P450. J Biochem Mol Toxicol. 2007;21(4):169–75.
de Wildt SN, Kearns GL, Leeder JS, van den Anker JN. Cytochrome P450 3A: ontogeny and drug disposition. Clin Pharmacokinet. 1999;37(6):485–505.
Stevens JC, Hines RN, Gu C, Koukouritaki SB, Manro JR, Tandler PJ, et al. Developmental expression of the major human hepatic CYP3A enzymes. J Pharmacol Exp Ther. 2003;307(2):573–82. doi:10.1124/jpet.103.054841.
Kuehl P, Zhang J, Lin Y, Lamba J, Assem M, Schuetz J, et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet. 2001;27(4):383–91. doi:10.1038/86882 86882.
Dally H, Bartsch H, Jager B, Edler L, Schmezer P, Spiegelhalder B, et al. Genotype relationships in the CYP3A locus in Caucasians. Cancer Lett. 2004;207(1):95–9. doi:10.1016/j.canlet.2003.12.011.
Gervasini G, Vizcaino S, Gasiba C, Carrillo JA, Benitez J. Differences in CYP3A5*3 genotype distribution and combinations with other polymorphisms between Spaniards and Other Caucasian populations. Ther Drug Monit. 2005;27(6):819–21.
Sinues B, Vicente J, Fanlo A, Vasquez P, Medina JC, Mayayo E, et al. CYP3A5*3 and CYP3A4*1B allele distribution and genotype combinations: differences between Spaniards and Central Americans. Ther Drug Monit. 2007;29(4):412–6. doi:10.1097/FTD.0b013e31811f390a.
Ameyaw MM, Regateiro F, Li T, Liu X, Tariq M, Mobarek A, et al. MDR1 pharmacogenetics: frequency of the C3435T mutation in exon 26 is significantly influenced by ethnicity. Pharmacogenetics. 2001;11(3):217–21.
Ostrovsky O, Nagler A, Korostishevsky M, Gazit E, Galski H. Genotype and allele frequencies of C3435T polymorphism of the MDR1 gene in various Jewish populations of Israel. Ther Drug Monit. 2004;26(6):679–84.
Santos PC, Soares RA, Santos DB, Nascimento RM, Coelho GL, Nicolau JC, et al. CYP2C19 and ABCB1 gene polymorphisms are differently distributed according to ethnicity in the Brazilian general population. BMC Med Genet. 2011;12:13. doi:10.1186/1471-2350-12-13.
ACKNOWLEDGMENTS
Jules Heuberger was supported by a fellowship of the Saal van Zwanenberg Stichting in the Netherlands.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sy, S.K.B., Heuberger, J., Shilbayeh, S. et al. A Markov Chain Model to Evaluate the Effect of CYP3A5 and ABCB1 Polymorphisms on Adverse Events Associated with Tacrolimus in Pediatric Renal Transplantation. AAPS J 15, 1189–1199 (2013). https://doi.org/10.1208/s12248-013-9528-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-013-9528-9