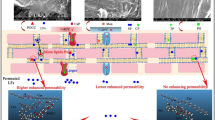
Abstract
The purpose of this study was to determine the ability and the safety of a series of alkylammonium C12-gemini surfactants to act as permeation enhancers for three model drugs, namely lidocaine HCl, caffeine, and ketoprofen. In vitro permeation studies across dermatomed porcine skin were performed over 24 h, after pretreating the skin for 1 h with an enhancer solution 0.16 M dissolved in propylene glycol. The highest enhancement ratio (enhancement ratio (ER) = 5.1) was obtained using G12-6-12, resulting in a cumulative amount of permeated lidocaine HCl of 156.5 μg cm−2. The studies with caffeine and ketoprofen revealed that the most effective gemini surfactant was the one with the shorter spacer, G12-2-12. The use of the latter resulted in an ER of 2.4 and 2.2 in the passive permeation of caffeine and ketoprofen, respectively. However, Azone was found to be the most effective permeation enhancer for ketoprofen, attaining a total of 138.4 μg cm−2 permeated, 2.7-fold over controls. This work demonstrates that gemini surfactants are effective in terms of increasing the permeation of drugs, especially in the case of hydrophilic ionized compounds, that do not easily cross the stratum corneum. Skin integrity evaluation studies did not indicate the existence of relevant changes in the skin structure after the use of the permeation enhancers, while the cytotoxicity studies allowed establishing a relative cytotoxicity profile including this class of compounds, single chain surfactants, and Azone. A dependence of the toxicity to HEK and to HDF cell lines on the spacer length of the various gemini molecules was found.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The stratum corneum (SC) is the skin's outermost layer and it is generally recognized to be the major barrier to the permeation of drugs applied topically. SC is composed of keratinocytes embedded in a complex extracellular lipid mixture, containing ceramides (45–50%), cholesterol (25%), free fatty acids (10–15%) and some other lipids such as cholesterol sulfate, highly organized as gel phase membrane domains (1).
The biggest challenge in transdermal drug delivery is to reversibly alter the barrier properties of the SC and, therefore, increase the transport of drugs across the skin. Such compounds are generally known as chemical penetration enhancers (CPE) and have different mechanisms of action, which include increasing the partition of the drugs to the skin or their diffusion rates in the SC (2–4).
According to Fick's law of diffusion (Eq. 1)
where J is the steady-state flux, D is the diffusion coefficient of the drug in the SC, h is the diffusional path length or, in this case, the membrane thickness, P is the partition coefficient, and C 0 is the drug concentration applied, a combination of enhancement effects on diffusivity and partitioning may result in an enhancement effect. Synergistic effects have been verified for many combinations, including Azone and propylene glycol (5).
Surfactants comprise a broad class of compounds with surface-active properties, and consequently have a great potential to be used as permeation enhancers. Surfactants are often employed in transdermal formulations to increase the permeation of drug molecules across the skin. At concentrations below the critical micelle concentration (cmc), surfactants exist free in solution, and the effects of increasing skin permeability are generally ascribed to the ability of surfactant molecules to penetrate in the hydrophobic domains of the SC, therefore increasing the fluidity of the lipid structures (6). The major concerns about the use of surfactants in transdermal drug delivery are associated with the possibility of local irritation, erythema, itching, and the time required for adequate skin recovery, as well as problems related with the transport into the systemic circulation (7–9).
Despite the fact that cationic surfactants are reputedly at least as irritating as anionic ones, they are used in cosmetic formulations and have been reported to possess antibacterial properties (10,11). The potential of some alkyl surfactants to act as chemical permeation enhancers for various drugs have been studied and reported in the literature (6,12–15). Some studies have focused on the influence of the alkyl chain length on the potency of the surfactants as chemical permeation enhancers. The highest enhancer activity was found for surfactants with C12 alkyl chains (16).
Unlike conventional surfactants that are composed of a single hydrophobic tail connected to a polar head group, gemini (or dimeric) surfactants structurally consist of at least two hydrophobic tails and two hydrophilic head groups covalently linked by a spacer group (17,18). The chemical structure of the spacer and head groups may vary, resulting in compounds with different physico-chemical properties. The spacer can be as short as two methylene groups or long (12 methylene groups), rigid (stylbene) or flexible (methylene chain), polar (polyether) or nonpolar (polyether sugar). Also, the polar head group can be positive (e.g. ammonium as in the compounds reported in this study), negative (phosphate, sulfate) or nonionic (aliphatic or aromatic) (18,19).
Gemini surfactants are typically micelle-forming surfactants that are reportedly more efficient in lowering the surface tension and have considerably lower cmc values than the corresponding single chain surfactants at the same temperature, generally by one order of magnitude or more. These interfacial properties are attributed to the higher hydrophobicity of the gemini compared to their single chain homologues; furthermore, it is observed that for a series of n–s–n compounds, those properties are non-monotonically influenced by the spacer length, s (11,20). In what concerns to skin penetration enhancement of drugs, the improved interfacial properties may result in an efficient penetration enhancement using lower molar concentrations, therefore reducing the undesired irritancy or toxic effects on the skin. In addition, alkylammonium cationic gemini surfactants similar to those used in the present study have been reported to significantly interact with lipid membranes. It was reported that the presence of these compounds decreased the degree of organization of dipalmitoylphosphatidylcholine bilayers. The effect on the liposomes was found to be dependent on the spacer length, with the vertical positioning of gemini molecules inside the bilayer being governed by the hydrophobic effect upon the spacer (21).
The objective of this study is to evaluate the potential of a series of cationic C12-alkylammonium gemini surfactants as CPE for various model drugs across porcine skin, following a methodology identical to that used in a previous work of the authors (22). These compounds consist of dimethylene-1,2-bis(dodecyldimethylammonium bromide), hexamethylene-1,2-bis(dodecyldimethylammonium bromide) and decamethylene-1,2-bis(dodecyldimethylammonium bromide), respectively, referred in this manuscript as G12-2-12, G12-6-12, and G12-10-12.
In spite of the fact that literature is abundant with studies that assess skin permeation enhancing by a variety of compounds, systematic approaches relating to structure and enhancing efficiency are rare. In this work, different spacer lengths were chosen, implying different cmc values and different hydrophobicity of the compounds. Single chain alkylammonium surfactant dodecyltrimethylammonium bromide (DTAB) and Azone, a well-known CPE (16,23–28), both having C12-akyl chains in their structures, were also used in this study, for comparative purposes (Fig. 1).
The variations imposed on the CPE are combined with the use of drugs with distinct physico-chemical properties: lidocaine HCl, positively charged; caffeine (non-ionized and hydrophilic) and ketoprofen (non-ionized and hydrophobic).
Additionally, the effects on the skin structure of the CPE and methodology employed was studied using light microscopy and scanning electron microscopy (SEM). The cytotoxicity of all compounds was also assessed and discussed. These studies were performed in cultured human epidermal keratinocytes (HEK) and in human dermal fibroblasts (HDF).
EXPERIMENTAL SECTION
Materials
Hydroxypropylmethyl cellulose (HPMC K15M) was a kind gift from Dow Chemical Company (USA). Lidocaine hydrochloride monohydrate, ketoprofen, caffeine, and DTAB (BioXtra, ≈99%), propylene glycol (PG) (Reagent Plus, 99%) were purchased from Sigma-Aldrich (Saint Louis, MO, USA). Azone was synthesized at the New Jersey Center for Biomaterials, Rutgers, The State University of New Jersey (Piscataway, NJ, USA). The three cationic gemini surfactants used in this study (Fig. 1), alkylene bis(dodecyldimethylammonium bromide), 12-s-12 for s = 2, 6, and 10, namely dimethylene-1,2- bis (dodecyldimethylammonium bromide), hexamethylene-1,2- bis (dodecyldimethylammonium bromide) and decamethylene-1,2- bis (dodecyldimethylammonium bromide), were synthesized and purified in the Department of Chemistry and Biochemistry, University of Porto, according to the method of Menger (17,29,30). The purity of the compounds was ascertained by NMR, differential scanning calorimetry and determination of cmc by surface tension. The values obtained for cmc were 0.85, 0.96, and 0.40 mM for G12-2-12, G12-6-12 and G12-10-12, respectively, all in good agreement with previously reported values (31). Furthermore, no dips near the cmc break were found in the surface tension vs. concentration plots, further confirming that surface-active impurities are absent. Phosphate buffer saline (PBS) tablets were purchased from TIC Gums (Belcamp, MD, USA). Formalin 10% was purchased from Fisher Scientific, Inc. (Torrance, CA, USA). CellTiter 96® AQueous One Solution Cell Proliferation Assay (MTS) was purchased from Promega Corp (Madison, WI, USA). Tissue-Tek® O.C.T™ Compound was purchased from Sakura Finetek USA, Inc. (Agawam, MA, USA). Porcine skin tissue was obtained from the back of young Yorkshire pigs (26.5–28 kg, UMDNJ, Newark, NJ). HEK and HDF were purchased from Invitrogen™ (Carlsbad, CA, USA).
Hydrogel Composition and Preparation
Drug-loaded HPMC hydrogels were prepared as follows. Lidocaine HCl hydrogel contained 2.5% (w/w) lidocaine HCl, 1% (w/w) HPMC K15M and deionized water. Caffeine hydrogel was composed of 1.5% caffeine, 1% (w/w) HPMC K15M and deionized water. Ketoprofen hydrogel contained 5% (w/w) ketoprofen, 1% (w/w) HPMC dissolved in a mixture of PG/water 80:20.
Porcine Skin Preparation
Porcine skin was excised and dermatomed using a Padgett® Model B Electric Dermatome (Integra LifeSciences, Plainsboro, NJ) with 650–750 μm thickness and stored at −80°C. Before the experiments, skin samples were thawed at room temperature, before immersion in PBS (pH = 7.4) for 1 h, for equilibration.
Composition and Preparation of the Enhancer Solutions
The enhancer solutions were prepared at a concentration of 0.16 M in PG. All compounds were soluble in PG at the concentration used, and produced clear solutions.
Drug Delivery Studies
In vitro permeation studies were performed over 24 h using vertical Franz diffusion cells (PermeGear, Inc., PA, USA) with a diffusion area of 0.64 cm2 and a receptor compartment of 5.1 mL filled with PBS solution (pH = 7.4). The receptor medium was maintained at 37°C ± 0.1 and stirred during the permeation experiments. Drug-loaded hydrogel (0.3 mL) was placed in each donor compartment and immediately covered with Parafilm®, preventing evaporation. Dermatomed porcine skin samples with a thickness of approximately 0.8 mm were used as a membrane. At predetermined time points, namely 0, 4, 8, 12, 16, 20, 22, and 24 h, 300 μL samples were collected from the receptor compartment and immediately replaced with 300 μL of new PBS solution. The samples were kept in the refrigerator prior to HPLC analysis. Before initiating the in vitro permeation experiments, all the skin samples, except the control, were pretreated with 60 μL of the various enhancer solutions, for 1 h. The time at zero, t = 0, corresponds to the instant of application of the drug-loaded hydrogel in the donor compartment. The sample concentrations were corrected to account for drug taken out at each sample point.
Drug Quantification
All samples were analyzed using HPLC. The system consisted of an Agilent HP 1100 series with an autosampler, equipped with a quaternary pump and a VMD detector and an Agilent ChemStation. The quantification of lidocaine hydrochloride was carried out using a reverse-phase Nova-Pak® C18 Column (4-mm pore size, 3.9 × 300 mm—Waters, Milford, MA, USA) with a guard column as the stationary phase. The mobile phase was prepared by mixing 50 mL of glacial acetic acid to 930 mL of water followed by adjusting the pH to 3.40 with a solution of 1-M NaOH. Later, four volumes of the previous solution were mixed with one volume of acetonitrile. The flow rate was set to 1.1 mL/min and the injection volume was 20 μL. Lidocaine hydrochloride was detected at 254 nm and the retention time was ca. 4.5 min. The method showed a linear response in the 0.025–1.0 mg/mL (R 2 = 0.9998) with a daily relative standard deviation (RSD) < ±3.0%. A reverse-phase C18 column (150 × 4.6 mm C18 (2) 100 A Luna 5 μm, Phenomenex®) with a guard column was the stationary phase for the caffeine assay, at 25°C. The mobile phase consisted of a mixture of 15% acetonitrile and 85% of a solution of 0.1% H3PO4 in water. The flow rate was set to 1.0 mL/min and the injection volume was 20 μL. Caffeine was detected at 273 nm and the retention time was ca. 4 min. The method showed a linear response in the 0.01–500 mg/mL range (R 2 = 0.9973) with a daily RSD < ±3.0%. Ketoprofen was detected at 256 nm using a reverse-phase C18 column (150 × 4.6 mm C18 (2) 100 A Luna 5 μm, Phenomenex®) with a guard column as the stationary phase at 25°C. The mobile phase consisted of a mixture of 45% of a 0.1% solution of H3PO4 and 55% acetonitrile. The flow rate was set to 1.0 mL/min and the injection volume was 20 μL. In these conditions, the method exhibited a linear response between 0.01–100 mg/mL (R 2 = 1.0000), and the retention time was close to 4 min with a daily RSD < ±3.0%.
Data Analysis in the Permeation Studies
The steady flux at time t (J, μg cm−2) was calculated from the slope of the linear portion of the plot of the cumulative drug amount permeated vs time. Q 24 refers to the cumulative drug amount present in the receptor compartment solution after 24 h of permeation experiment. The enhancement ratio (ER) for flux was calculated using
Results are presented as mean ± standard deviation (SD) (N), where N is the number of replicates. Flux values were examined for significance using unpaired Student's t test. The p value was set to 0.05, meaning that for p < 0.05, there was statistically significant difference between the control and the enhancers tested.
Skin Integrity Evaluation
Skin samples used in the permeation studies were subsequently rinsed with deionized water, dried and stored at −80°C. These samples were later investigated for the occurrence of morphological changes caused by the CPE and methodology employed.
Histology—Light Microscopy Studies
Skin samples used in the permeation studies were sectioned and fixed using 10% buffered formalin for 24 h at room temperature. Skin samples were dehydrated, embedded in Tissue-Tek® O.C.T. compound and frozen (32). Cross-section slices of 7 μm thickness were obtained using a microtome (Leica Model CM 1850, Leica Microsystems, Inc. Bannockburn, IL, USA) and stained following the Ellis Hematoxylin and Eosin (H&E) staining protocol (33).
The stained slices were analyzed using a Nikon Eclipse E 800 light microscope (Micro Optics, Cedar Knolls, NJ, USA) at ×10, ×20, and ×40. A Nikon Digital Camera (Model DXM 1200) was used to capture images. Images were processed using SPOT TM Imaging Software, Version 5.0 (Diagnostic Instrument, Inc., Sterling Heights, MI, USA).
Scanning Electron Microscopy Studies
Skin samples were submerged in Tissue-Tek® O.C.T. Compound and frozen. Cross-sectional slices of the frozen samples were prepared (10-μm thickness) using a microtome (Leica Model CM 1850, Leica Microsystems, Inc. Bannockburn, IL, USA). The samples were defrosted and fixed using 4% formaldehyde for 1.5 h. Subsequently, skin samples were rinsed and placed in deionized water, at room temperature for 2 h and dehydrated. The dehydrated samples were dried with a critical-point drier (model CPD 020) and coated with gold. Surface and cross-sections pictures of the samples were taken using Scanning Electron Microscopy (SEM - model AMARY-18301).
Cytotoxicity Studies—MTT/MTS Assay
MTT/MTS assays are a colorimetric method used to determine the number of viable cells in cytotoxicity assays, widely reported in the literature for similar systems (34–37). MTS reagent (CellTiter 96® AQueous One Solution Reagent) contains a tetrazolium compound [(3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt] which is converted by mitochondrial dehydrogenase enzymes of living cells into a colored formazan compound. The number of viable cells is directly proportional to the quantity of formazan product formed as measured by the absorbance at 490 nm (Promega Corp. Protocol, 2009). The levels of formazan formed were quantified using a Microplate Power Wave X Scanning Spectrophotometer (Bio-TEK Instruments, Inc. Winooski, VT, USA).
Cytotoxicity studies were carried out both in cultured HEK and in HDF seeded at 8,000 cells/well in 96-well plates under sterile conditions. The objective was to evaluate the toxic effects of the CPE on these types of skin cells, establishing a concentration-toxicity response. HEK were seeded in 100 μL/well of Epilife® (MEPI 500CA) medium supplemented with gentamicin (Gibco®), human keratinocyte growth supplement (Gibco®) and were incubating for 24 h at 37°C, 5% CO2, and 90% R.H. HDF were seeded in 100 μL/well of GIBCO®- Dulbecco's Modified Eagle Medium with 100 IU/mL penicillin, 100 IU/mL streptomycin, and 10% fetal bovine serum. All the media and biological reagents were purchased directly from Invitrogen™, Inc. (Carlsbad, CA, USA).
After seeding, the culture medium was removed and cells were exposed to medium (control), PG, and various concentrations (S1-160, S2-16, S3-1.6, S4-0.16, S5-0.016 μM) of Azone and cationic gemini surfactants solutions in PG, subsequently diluted in culture medium and placed in the incubator for an additional period of 24 h in the same environmental conditions. After exposure, testing solutions were removed and replaced by a pre-mixed solution of MTS reagent (CellTiter 96® AQueous One Solution) and appropriate medium (Promega Corp. Protocol, 2009), and were returned to the incubator for 2 h. The 96-well plates were read at 490 nm using a plate reader (Microplate Power Wave X Scanning Spectrophotometer—Bio-TEK Instruments, Inc. Winooski, VT, USA).
Results are presented as percent cell viability (mean ± standard deviation, N = 6), C.V., after background absorbance (abs), determined from “no cell” control wells was subtracted, according to Eq. 3.
RESULTS AND DISCUSSION
In Vitro Permeation Studies: Transdermal Delivery of Lidocaine Hydrochloride, Caffeine, and Ketoprofen
As described in the experimental section, three gemini C12 surfactants, DTAB, and Azone were dissolved in PG at a concentration of 0.16 M. The enhancer solutions were applied to the epidermal side of dermatomed porcine skin placed between the donor and receptor compartments of Franz diffusion cells for 1 h prior to the application of the lidocaine HCl loaded hydrogels. The results obtained are presented in Table I.
The use of all three gemini surfactants tested, DTAB, and Azone solutions resulted in a statistically significant increase (p < 0.05) of lidocaine HCl permeation, when compared to control. G12-6-12 was shown to be the most effective permeation enhancer resulting in a fivefold increase of the ER, followed by G12-10-12 (ER = 3.9), DTAB (ER = 3.7), and Azone (ER = 3.4). In contrast, PG and the gemini surfactant with the shorter spacer (G12-2-12) showed poor performance.
Caffeine is a nonionic hydrophilic compound. The results obtained with this agent (see Table I) and show that PG significantly decreased the amount of drug permeated, to approximately one half compared to the control. In contrast, gemini surfactants G12-2-12 and G12-6-12 and DTAB significantly increased the caffeine permeated after 24 h by approximately twofold. Surprisingly, Azone had a modest performance in terms of penetration enhancement, causing only a slight increase of caffeine permeated, with a value that is not statistically different from control.
Permeation experiments using ketoprofen as model drug were slightly different from those of lidocaine HCl and caffeine. Unlike in the case of the previous drug-loaded hydrogels, PG was directly incorporated in the hydrogel formulation due to low solubility (51 mg/L) of ketoprofen in water-based vehicles and, for this reason, was not tested as CPE separately. Permeation data (presented in Table I and Fig. 2-c) shows that Azone and Gemini G12-2-12 were the most effective CPE with 2.7 and 2.2 enhancement ratios, respectively, and the only CPE tested that proved to be statistically different from the control.
Gemini G12-10-12 (ER = 1.3) and DTAB (ER = 1.4) caused a mild increase in the ketoprofen permeated, while the use of gemini G12-6-12 resulted in the same amount of ketoprofen permeated as control.
Overview and Comparison
This work indicates that the most effective gemini are those with shorter or intermediate spacer lengths. In the case of lidocaine HCl, the maximum ER was found with a spacer of six carbons (G12-6-12, ER = 5.1), and the CPE with the shorter spacer (G12-2-12) was the least effective. However, as the hydrophobicity of the drug increases, the trend changes; in the case of caffeine and ketoprofen, G12-2-12 was clearly the most effective CPE accounting for a 2.5-fold and 2.2-fold increase in the flux, respectively. In studies with caffeine, the effect of G12-6-12 was still significant (ER = 1.8) while that of G12-10-12 was found negligible. Also negligible were the effects of both G12-6-12 and G12-10-12 in the case of ketoprofen.
The results obtained indicate that the effect of CPE is especially important in cases where the drug is clearly hydrophilic, decreasing as the hydrophobicity of the drug increases. This trend parallels with a previous work in which terpenes were used as CPE for drugs with different hydrophobicities (38).
Recent studies on the effect of gemini surfactants on lipid bilayers show that gemini molecules with tails containing 12 carbons are effective in inducing a decrease in the overall order of the bilayers due to an interplay of effects (21). It is well-known that the interfacial and self-assembling properties of G12-s-12 gemini surfactants are determined by spacer length in a subtle way (39). The existence of an optimal spacer (and thus charge-to-charge) distance in the interaction of gemini with other molecules has been reported before, for example in gemini–polyelectrolyte mixtures (40). It was found that the longer spacers tend to bend towards the inside of the bilayers, just as in neat micelles and at air–water interface, which may explain, as a first approach, the similitude of action found in the present work between G12-6-12 and G12-10-12. In contrast, the short-spacer gemini seems to be the most efficient enhancer for caffeine and ketoprofen. Even though these drugs are uncharged, here, we cannot discard the presence of electrostatic effects (albeit weak) promoting the enhancement, since caffeine is a relatively polar molecule and ketoprofen has an ionizable acidic group, and in both cases, interaction with a cationic head group should be favorable. One slightly surprising result is the fact that G12-10-12, the most hydrophobic of the gemini, is not particularly effective as an enhancer for the most hydrophobic drug (ketoprofen), while G12-2-12 is significantly better.
The present results indicate that the enhancing effects for the gemini molecules are a result of a combination of mechanisms, probably disturbing the SC lipid organization but also promoting the transport of the more hydrophilic, and charged drugs.
Effects Upon the Skin and Cytotoxicity Studies
Potential effects on the skin and morphological changes caused by the use of the chemical penetration enhancers and formulations were evaluated by means of histology-light microscopy and SEM. By comparing the images from the untreated skin samples and images of skin treated with the CPE, it is observed that there are not relevant macroscopic differences concerning the integrity and cohesion between the skin layers (see Fig. 3).
Untreated (control) porcine skin pictures were captured using an optical light microscope at ×20 (a) and SEM at ×400 (c). The distinct skin layers can be observed: stratum corneum (SC), viable epidermis (VE), and dermis (d). The skin samples used in the permeation experiments that were previously pretreated with the CPE were also evaluated and compared. An example of skin pretreated with the Gemini 12-10-12 is depicted at b and d. The pretreated skin samples resemble untreated skin (control)
SEM studies were also performed in order to observe the occurrence of potential harmful effects caused by the use of CPE on the skin that could not be perceived using conventional light microscopy. It can be observed that some stratum corneum cells are naturally shedding, but it is seen both in the control and treated samples. Changes or damage to the skin structure caused by the CPE used that could result in permanent alterations or loss of integrity are not detected. These observations clearly contrast with a positive control in which the detachment of corneocytes is usually visible, as shown in previous work (41).
Skin irritation is commonly defined as a reversible inflammatory reaction produced by the arachidonic acid cascade and cytokines in the viable skin cells like keratinocytes and fibroblasts.
The determination of the cytotoxicity of the CPE used in this work was carried out by exposing cultured HEK and HDF to various concentrations of the CPE, following the MTS assay protocol. MTT/MTS assay is generally regarded as a method that is able to provide a cost-effective, precise, and reproducible index of viability for skin cells and an alternative for skin irritation assessment (42–44). Unlike the skin tissue, the groups of cells cultured for the MTS assay is noticeably less complex in number and in cell types when compared to the skin tissue. Therefore, the results should be regarded as an indication of potential and relative toxicity and irritation of the compounds to the skin cells. Note that the concentration values used in the cytotoxicity assays are clearly below that used for the pretreatment of the skin. While the former values are established on the basis of the viability results, only a fraction of the latter reaches the viable regions of the skin.
Standard plots for HEK (R 2 = 0.986) and for HDF (R 2 = 0.978) were created based on the absorbance readings at 490 nm for 0, 2.000, 4.000, 6.000, 8.000, and 10.000 cells/well in order to ensure the linearity of the MTS assay method used. The results obtained in the MTS assay are displayed in Fig. 4 (HEK on top and HDF on the bottom).
As shown in Fig. 4, PG did not significantly reduce the viability of HEK when compared to the control (cells not subjected to any CPE exposure). Therefore, it can be assumed that the effect on the HEK viability is caused by the CPE and not by the vehicle where the CPE was incorporated. In opposite, when a concentration of 160 μM (S1) was applied to the cells it was observed that approximately 100% cell death occurred in all cases. The exposure to a concentration of 16 μM (S2) produced different results, depending on the CPE used. HEK showed higher tolerance to the presence of DTAB, than for any gemini surfactants used or Azone. It can also be concluded that concentrations of 1.6 μM or lower (S3, S4, and S5) are not considered harmful to the cells, as the cell viability is always close to 100%.
The results obtained with HDF demonstrated that HDF showed higher tolerance to the presence of the CPE than HEK, as the cell viability was respectively higher for the first and lower for the latter, for the same concentration of CPE employed.
PG exposure resulted in a slight reduction in HDF viability, yet not statistically significant from the control, as expected. CPE concentrations of 160 μM (S1) caused cell death in all cases except for gemini surfactant G12-2-12, where an approximately 60% of viability was observed. The presence of a concentration of 16 μM (S2) or lower of any penetration modifier did not cause a significant decrease in the HDF viability, except for enhancers G12-6-12 and G12-10-12. In both cases, cell viability was reduced in approximately 50% when HDF were exposed to a concentration of 16 μM (S2) and slightly less for a concentration of 1.6 μM (S3) only observed for G12-6-12.
A global analysis of the MTS assay results obtained with HDF suggests that the cytotoxicity of the gemini surfactants studied is dependent on spacer length. The compound with the shortest spacer, G12-2-12, was found to be less toxic to HDF when compared to the other gemini and, in fact, to all CPE. We recall that both electrostatic (head group charge density) and packing effects (spacer conformation and vertical positioning on the bilayer) may lie behind the structural perturbation of membranes that cause cytotoxic effects. A possible explanation for G12-2-12 effects in cultured HDF is that this gemini, as previously mentioned, may easily fit into the lipid bilayer structure more due to its cylindrical geometric shape (more akin to that of lipid molecules), thus causing less structural perturbation on the membrane. This effect, however, was not noticed in HEK, where DTAB was found to be the least destructive CPE, suggesting that for this cell line electrostatic effects (higher head group charge density for 12-2-12) may play a bigger role. Nevertheless, even for HEK, the shortest spacer gemini seems to be the least cytotoxic of all the Gemini compounds, and even a little more effective than Azone.
CONCLUSIONS
This work presents a systematic approach to the use of a series of gemini surfactants as permeation enhancers for drugs of varying hydrophobicity and belonging to different therapeutic groups. The gemini surfactant molecules differ only in the spacer length, comprising 2, 6, and 10 carbon atoms. It was observed that a maximal enhancing effect was achieved with the most hydrophilic and charged drug (lidocaine HCl). In this case, the gemini surfactant with the intermediate spacer length was the most effective. In contrast, for caffeine and ketoprofen, G12-2-12 was the most effective CPE. This suggests that this class of molecules promotes both SC perturbation and drug transport through the lipid barrier. It was also found that these compounds possess similar cytotoxic profiles to that of Azone and their direct use in the skin tissue did not determine significant morphological changes. They are, therefore, promising CPE candidates, essentially directed at permeation enhancing of hydrophilic drugs. Further tests should explore other types of gemini surfactants, such as those based on amino acids.
References
Madison KC. Barrier function of the skin: "La Raison d'Être" of the epidermis. J Invest Dermatol. 2003;121(2):231–41.
Finnin BC, Morgan TM. Transdermal penetration enhancers: applications, limitations, and potential. J Pharm Sci. 1999;88(10):955–8.
Asbill CS, Michniak BB. Percutaneous penetration enhancers: local versus transdermal activity. Pharm Sci Technol To. 2000;3(1):36–41.
Bach M, Lippold BC. Percutaneous penetration enhancement and its quantification. Eur J Pharm Biopharm. 1998;46(1):1–13.
Wotton PK, Møllgaard B, Hadgraft J, Hoelgaard A. Vehicle effect on topical drug delivery. III. Effect of Azone on the cutaneous permeation of metronidazole and propylene glycol. Int J Pharm. 1985;24(1):19–26. doi:10.1016/0378-5173(85)90141-3.
Walters KA, Bialik W, Brain KR. The effects of surfactants on penetration across the skin. Int J Cosmet Sci. 1993;15(6):260–71.
Effendy I, Maibach HI. Surfactants and experimental irritant contact dermatitis. Contact Dermatitis. 1995;33(4):217–25. doi:10.1111/j.1600-0536.1995.tb00470.x.
Naik A, Kalia YN, Guy RH. Transdermal drug delivery: overcoming the skin's barrier function. Pharm Sci Technol To. 2000;3(9):318–26.
Scheindlin S. Transdermal drug delivery: past, present, future. Mol Interv. 2004;4(6):308–12. doi:10.1124/mi.4.6.1.
Attwood D, Florence AT. Surfactant systems: their chemistry, pharmacy, and biology. London: Chapman and Hall; 1983.
Shukla D, Tyagi VK. Gemini surfactants: a review. J Oleo Sci. 2006;55(8):381–90.
Tan EL, Lid J-C, Chien YW. Effect of cationic surfactants on the transdermal permeation of ionized indomethacin. Drug Dev Ind Pharm. 1993;19(6):685–99. doi:10.3109/03639049309062975.
Babu R, Mandip S, Narayanasamy K. Structure-activity relationship of chemical penetration enhancers. Percutaneous Penetration Enhancers. 2nd ed. Informa Healthcare; 2005. p. 17-33.
Ashton P, Walters KA, Brain KR, Hadgraft J. Surfactant effects in percutaneous absorption I. Effects on the transdermal flux of methyl nicotinate. Int J Pharm. 1992;87(1–3):261–4. doi:10.1016/0378-5173(92)90251-v.
Kitagawa S, Kitagawa S, Kasamaki M, Ikarashi A. Effects of n-alkyltrimethylammonium on skin permeation of benzoic acid through excised guinea pig dorsal skin. Chem Pharm Bull. 2000;48:1698–701.
López A, Llinares F, Cortell C, Herráez M. Comparative enhancer effects of Span®20 with Tween®20 and Azone® on the in vitro percutaneous penetration of compounds with different lipophilicities. Int J Pharm. 2000;202(1–2):133–40.
Menger FM, Littau CA. Gemini-surfactants: synthesis and properties. J Am Chem Soc. 1991;113(4):1451–2.
Menger FM, Keiper JS. Gemini surfactants. Angew Chem Int Edit. 2000;39(11):1906–20.
Laschewsky A, Lunkenheimer K, Rakotoaly RH, Wattebled L. Spacer effects in dimeric cationic surfactants. Colloid Polym Sci. 2005;283(5):469–79. doi:10.1007/s00396-004-1219-8.
Zana R. Dimeric (gemini) surfactants: effect of the spacer group on the association behavior in aqueous solution. J Colloid Interf Sci. 2002;248(2):203–20. doi:10.1006/jcis.2001.8104.
Almeida JAS, Marques EF, Jurado AS, Pais AACC. The effect of cationic gemini surfactants upon lipid membranes. An experimental and molecular dynamics simulation study. Phys Chem Chem Phys. 2010;12(43):14462–76.
Silva SMC, Hu L, Sousa JJS, Pais AACC, Michniak-Kohn BB. A combination of nonionic surfactants and iontophoresis to enhance the transdermal drug delivery of ondansetron HCl and diltiazem HCl. Eur J Pharm Biopharm. 2012;80(3):663–73. doi:10.1016/j.ejpb.2011.11.010.
Harrison JE, Groundwater PW, Brain KR, Hadgraft J. Azone® induced fluidity in human stratum corneum. A fourier transform infrared spectroscopy investigation using the perdeuterated analogue. J Control Release. 1996;41(3):283–90.
Degim IT, Uslu A, Hadgraft J, Atay T, Akay C, Cevheroglu S. The effects of Azone and capsaicin on the permeation of naproxen through human skin. Int J Pharm. 1999;179(1):21–5.
Lambert WJ, Higuchi WI, Knutson K, Krill SL. Dose-dependent enhancement effects of Azone on skin permeability. Pharm Res. 1989;6(9):798–803. doi:10.1023/a:1015931715829.
Hou S, Flynn G. Enhancement of hydrocortisone permeation of human and hairless mouse skin by 1-dodecylazacycloheptan-2-one. J Invest Dermatol. 1989;93(6):774–9.
Sugibayashi K, Hosoya K, Morimoto Y, Higuchi W. Effect of the absorption enhancer, Azone, on the transport of 5-fluorouracil across hairless rat skin. J Pharm Pharmacol. 1985;37(8):578–80.
Agrawala P, Ritschel W. Influence of 1-dodecylhexahydro-2H-azepin-2-one (Azone) on the in vitro permeation of verapamil hydrochloride across rat, hairless mouse, and human cadaver skin. J Pharm Sci. 1988;77(9):776–8.
Menger FM, Littau CA. Gemini surfactants: a new class of self-assembling molecules. J Am Chem Soc. 1993;115(22):10083–90. doi:10.1021/ja00075a025.
Menger FM, Mbadugha BNA. Gemini surfactants with a disaccharide spacer. J Am Chem Soc. 2001;123(5):875–85. doi:10.1021/ja0033178.
Alami E, Beinert G, Marie P, Zana R. Alkanediyl-.alpha., omega.-bis(dimethylalkylammonium bromide) surfactants. 3. Behavior at the air-water interface. Langmuir. 1993;9(6):1465–7. doi:10.1021/la00030a006.
Hoppert M. Microscopic techniques in biotechnology. Microscopic techniques in biotechnology. Veinheim: Wiley-VCH; 2003. p. 114.
Ellis R. Ellis Hematoxylin and Eosin (H&E) Staining Protocol. 2010 [cited 2010]; Available from: http://www.ihcworld.com/_protocols/special_stains/h&e_ellis.htm. Accessed 14 Sept 2010.
Arechabala B, Coiffard C, Rivalland P, Coiffard LJM, Roeck-Holtzhauer YD. Comparison of cytotoxicity of various surfactants tested on normal human fibroblast cultures using the neutral red test, MTT assay and LDH release. J Appl Toxicol. 1999;19(3):163–5.
Eun HC, Chung JH, Jung SY, Cho KH, Kim KH. A comparative study of the cytotoxicity of skin irritants on cultured human oral and skin keratinocytes. Brit J Dermatol. 1994;130(1):24–8.
Korting HC, Schindler S, Hartinger A, Kerscher M, Angerpointner T, Maibach HI. MTT-assay and neutral red release (NRR)-assay: relative role in the prediction of the irritancy potential of surfactants. Life Sci. 1994;55(7):533–40.
Song Y, Xiao C, Mendelsohn R, Zheng T, Strekowski L, Michniak B. Investigation of iminosulfuranes as novel transdermal penetration enhancers: enhancement activity and cytotoxicity. Pharm Res. 2005;22(11):1918–25.
El-Kattan AF, Asbill CS, Kim N, Michniak BB. The effects of terpene enhancers on the percutaneous permeation of drugs with different lipophilicities. Int J Pharm. 2001;215(1-2):229–40.
Zana R. Dimeric and oligomeric surfactants. Behavior at interfaces and in aqueous solution: a review. Adv Colloid Interfac. 2002;97(1–3):205–53. doi:10.1016/S0001-8686(01)00069-0.
Burrows HD, Tapia MJ, Silva CL, Pais AACC, Fonseca SM, Pina J, et al. Interplay of electrostatic and hydrophobic effects with binding of cationic gemini surfactants and a conjugated polyanion: experimental and molecular modeling studies. The Journal of Physical Chemistry B. 2007;111(17):4401–10. doi:10.1021/jp070100s.
Wang Y, Fan Q, Song Y, Michniak B. Effects of fatty acids and iontophoresis on the delivery of midodrine hydrochloride and the structure of human skin. Pharm Res. 2003;20(10):1612–8.
Klein M, Shaw D, Barese S, Chapo G, Cuono C. A reliable and cost-effective in vitro assay of skin viability for skin banks and burn centers. J Burn Care Rehabil. 1996;17(6 Pt 1):565–70.
Watanabe T, Hasegawa T, Takahashi H, Ishibashi T, Itagaki H, Sugibayashi K. Utility of MTT assay in three-dimensional cultured human skin model as an alternative for draize skin irritation test: approach using diffusion law of irritant in skin and toxicokinetics-toxicodynamics correlation. Pharm Res. 2002;19(5):669–75.
Morikawa N, Kitagawa T, Tomihata K, editors. Assessment of the in vitro skin irritation of chemicals using the Vitrolife-Skin™ human skin model. Proc 6th World Congress on Alternatives & Animal Use in the Life Sciences; 2007; Tokyo, Japan: AATEX.
Acknowledgments
S.M.C. Silva acknowledges Fundação para a Ciência e a Tecnologia, Lisboa (Portugal), for Ph.D. Grant reference SFRH/BD/30537/2006. Partial funding provided by the Center for Dermal Research/NJ Center for Biomaterials, Rutgers-The State University of New Jersey www.centerfordermalresearch.org. The authors acknowledge Dr. Vishwas Rai for the help and assistance provided with the cell culture work. E.F. Marques gratefully acknowledges financing from FEDER/COMPETE and F.C.T. under project PTDC/QUI-QUI/115212/2009, and also Dr. Yujie Wang for the synthesis of the gemini surfactants.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Silva, S.M.C., Sousa, J.J.S., Marques, E.F. et al. Structure Activity Relationships in Alkylammonium C12-Gemini Surfactants Used as Dermal Permeation Enhancers. AAPS J 15, 1119–1127 (2013). https://doi.org/10.1208/s12248-013-9518-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-013-9518-y