Abstract
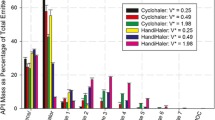
This study evaluated the effect of inhaled volume and simulated inspiratory flow rate ramps on fine particle output from dry powder inhalers (DPIs). A simple, robust system was developed to account for “rate of rise” (ramp) effects while maintaining a constant air flow through a multi-stage liquid impinger (MSLI), used for sizing the emitted particles. Ramps were programmed to reach 30 and 60 L/min over 100 milliseconds; 500 milliseconds; and 1, 2, and 3 seconds. Rotahaler was chosen as the test DPI. Testing was done with simulated inhalation volumes of 2 L and 4 L. Testing was also carried out using the USP apparatus 4. At 30 L/min, for a 2 L volume, the amount of drug exiting the device in fine particle fraction (FPF) increased from 2.33 μg to 6.04 μg from the 3-second ramp to the 100-millisecond ramp, with 11.64 μg in FPF for the USP (no ramp) method. At the same flow rate, for a 4 L volume, FPF increased from 2.23 μg to 8.45 μg, with 10.25 μg for the USP method. At 60 L/min, similar trends were observed. In general, at both flow rates, an increase in FPF was noted going from the shallowest to the steepest ramp. However, there were no significant differences in FPF when a 2 L inhaled volume was compared with a 4 L volume at each flow rate. Overall, these data suggest that the existing USP apparatus may overestimate FPF at flow rates lower than those recommended by the USP.
Similar content being viewed by others
References
Crompton GK. Dry powder inhalers: advantages and limitations. J Aerosol Med. 1991;4(3):151–156.
Dalby RN, Tiano SL. Medical devices for the delivery of therapeutic aerosols to the lung In: Hickey AJ, ed. Inhalation Aerosols: Physical and Biological Basis for Therapy. New York, NY: Marcel Dekker; 1996:441–473.
Ganderton D, Kassem NM. Dry powder inhalers. Adv Pharm Sci. 1992;6:165–191.
Kassem NM Ganderton D. The influence of carrier surface on the characteristics of inspirable powder aerosols. J Pharm Pharmacol. 1990;42:11P.
Prime D., Atkins, P., Slater A. et al Review of dry powder inhalers. Advanced Drug Delivery Reviews. 1997;26:51–58.
Newman SP, Johnson MA, Clarke SW. Effect of particle size of bronchodilator aerosols on lung distribution and pulmonary function in patients with chronic asthma [letter]. Thorax. 1988;43(2):159.
Hickey J, Concessio N, Platz R. Factors influencing the dispersion of dry powders as aerosols. Pharm Tech. 1994;18:58–64.
United States Pharmacopeia. Physical tests and determinations, Section 601: Aerosols. Vol 24. Philadelphia, PA: National Publishing; 2000:1895–1912.
Byron PR. Compendial dry powder testing: USP perspectives. Respiratory Drug Delivery IV. Deerfield, IL: Interpharm Press; 1994:153–162.
Stimuli to the revision process. Pharmacopeial Forum. 1997;23(6):5216.
Chavan VS, Dalby RN. Effect of rise in simulated inspiratory flow rate and carrier particle size on powder emptying from dry powder inhalers. AAPS Pharm Sci. 2000;2(2), article 10; 1–7 Available at http://www.aapspharmsci.org/scientificjournals/pharmsci/journal/10.html
Hickey AJ. Methods of aerosol particle size characterization. In: Hickey AJ, ed. Pharmaceutical Inhalation Aerosol Technology. Vol. 54, New York, NY: Marcel Dekker; 1992:219–255.
Washington C. Particle size analysis in inhalation metered dose inhaler technology. In: Purewal TS, Grant DJ, ed Metered Dose Inhaler Technology. Buffalo Grove, IL: Interpharm Press: 1998:177–145.
Miller NC, Olson BA, Marple VA. Measurement of the time required to reach steady state for air flow through some cascade impactors. Pharm Res. 1993;10(10):S165.
Webb J. In vitro testing of aerosols: what does the future hosd? J Aerosol Med. 1998;11(Suppl 1):S9-S10.
Newman SP. How well do in vitro particle size measurements predict drug delivery in vivo? J Aerosol Med. 1998;11(Suppl 1):S97-S104.
Staniforth JN. Particle interactions in dry powder formulation of aerocolloidal suspensions. In: Byron PR, ed Respiratory Drug Delivery II. Keystone, CO: Interpharm Press; 1990:531–542.
Author information
Authors and Affiliations
Additional information
Published: April 11, 2002
Rights and permissions
About this article
Cite this article
Chavan, V., Dalby, R. Novel system to investigate the effects of inhaled volume and rates of rise in simulated inspiratory air flow on fine particle output from a dry powder inhaler. AAPS PharmSci 4, 6 (2002). https://doi.org/10.1208/ps040211
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/ps040211