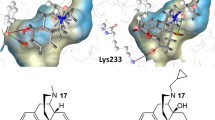
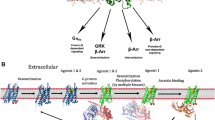
Abstract
Opioid receptors interact with a variety of ligands, including endogenous peptides, opiates, and thousands of synthetic compounds with different structural scaffolds. In the absence of experimental structures of opioid receptors, theoretical modeling remains an important tool for structurefunction analysis. The combination of experimental studies and modeling approaches allows development of realistic models of ligand-receptor complexes helpful for elucidation of the molecular determinants of ligand affinity and selectivity and for understanding mechanisms of functional agonism or antagonism. In this review we provide a brief critical assessment of the status of such theoretical modeling and describe some common problems and their possible solutions. Currently, there are no reliable theoretical methods to generate the models in a completely automatic fashion. Models of higher accuracy can be produced if homology modeling, based on the rhodopsin X-ray template, is supplemented by experimental structural constraints appropriate for the active orinactive receptor conformations, together with receptor-specific and ligand-specific interactions. The experimental constraints can be derived from mutagenesis and cross-linking studies, correlative replacements of ligand and receptor groups, and incorporation of metal binding sites between residues of receptors or receptors and ligands. This review focuses on the analysis of similarity and differences of the refined homology models of μ, δ, and κ-opioid receptors in active and inactive states, emphasizing the molecular details of interaction of the receptors with some representative peptide and nonpeptide ligands, underlying the multiple modes of binding of small opiates, and the differences in binding modes of agonists and antagonists, and of peptides and alkaloids.
Similar content being viewed by others
References
Kieffer BL. Recent advances in molecular recognition and signal transduction of active peptides: receptors for opioid peptides.Cell Mol Neurobiol. 1995;15:615–635.
Waldhoer M, Bartlett SE, Whistler JL. Opioid receptors.Annu Rev Biochem. 2004;73:953–990.
Pasternak GW. Multiple opiate receptors: déjà vu all over again.Neuropharmacology. 2004;47:312–323.
Vaccarino AL, Kastin AJ. Endogenous opiates: 2000.Peptides. 2001;22:2257–2328.
Hruby VJ, Agnes RS. Conformation-activity relationships of opioid peptides with selective activities at opioid receptors.Biopolymers. 1999;51:391–410.
Mansour A, Hoversten MT, Taylor LP, Watson SJ, Akil H. The cloned mu, delta and kappa receptors and their endogenous ligands: evidence for two opioid peptide recognition cores.Brain Res. 1995;700:89–98.
Mosberg HI, Hurst R, Hruby VJ, et al. Conformationally constrained cyclic enkephalin analogs with pronounced delta opioid receptor agonist selectivity.Life Sci. 1983;32:2565–2569.
Pelton JT, Gulya K, Hruby VJ, Duckles S, Yamamura HI. Somatostatin analogs with affinity for opiate receptors in rat brain binding assay.Peptides. 1985;6:159–163.
Pelton JT, Kazmierski W, Gulya K, Yamamura HI, Hruby VJ. Design and synthesis of conformationally constrained somatostatin analogues with high potency and specificity for mu opioid receptors.J Med Chem. 1986;29:2370–2375.
Kawasaki AM, Knapp RJ, Walton A et al. Syntheses, opioid binding affinities, and potencies of dynorphin A analogues substituted in positions 1, 6, 7, 8 and 10.Int J Pept Protein Res. 1993;42:411–419.
Meyer JP, Collins N, Lung FD, et al. Design, synthesis, and biological properties of highly potent cyclic dynorphin A analogues: analogues cyclized between positions 5 and 11J Med Chem. 1994;37:3910–3917.
Arttamangkul S, Murray TF, DeLander GE, Aldrich JV. Synthesis and opioid activity of conformationally constrained dynorphin A analogues. 1. Conformational constraint in the “message” sequence.J Med Chem. 1995;38:2410–2417.
Lung FD, Collins N, Stropova D, et al. Design, synthesis, and biological activities of cyclic lactam peptide analogues of dynorphin A(1-11)-NH2.J Med Chem. 1996;39:1136–1141.
Eguchi M. Recent advances in selective opioid receptor agonists and antagonists.Med Res Rev. 2004;24:182–212.
Okada T, Ernst OP, Palczewski K, Hofmann KP. Activation of rhodopsin: new insights from structural and biochemical studies.Trends Biochem Sci. 2001;26:318–324.
Li J, Edwards PC, Burghammer M, Villa C, Schertler GF. Structure of bovine rhodopsin in a trigonal crystal form.J Mol Biol. 2004;343:1409–1438.
Flower DR. Modeling G-protein-coupled receptors for drug design.Biochim Biophys Acta 1999;1422:207–234.
Flippen-Anderson JL, George C, Bertha CM, Rice KC. X-ray structure of potent opioid receptor ligands: etonitazene, cis-(+)-3-methylfentanyl, etorphine, diprenorphine, and buprenorphine.Heterocycles. 1994;39:751–766.
Urbanczyk-Lipkowska Z, Etter MC, Lipkowski AW, Portoghese PS. The crystal structure of a bimorphinan with highly selective kappa opioid receptor antagonist activity.J Mol Struct. 1987;159:287–295.
Griffin JF, Larson DL, Portoghese PS. Crystal structures of alpha-and beta-funaltrexamine: conformational requirement of the fumaramate moiety in the irreversible blockage of mu opioid receptorsJ Med Chem. 1986;29:778–783.
Calderon SN, Rice KC, Rothman RB, et al. Probes for narcotic receptor mediated phenomena. 23. Synthesis, opioid receptor binding, and bioassay of the highly selective delta agonist (+)-4-[(alpha R)-alpha-((2S,5R)-4-Allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,N-diethylbenzamide (SNC 80) and related novel nonpeptide delta opioid receptor ligands.J Med Chem. 1997;40:695–704.
Lavecchia A, Greco G, Novellino E, Vittorio F, Ronsisvalle G. Modeling of kappa-opioid receptor/agonists interactions using pharmacophore-based and docking simulations.J Med Chem. 2000;43:2124–2134.
Doi M, Ishida T, Inoue M. Conformational characteristics of opioid kappa-receptor agonist: crystal structure of (5S, 7S, 8S)-(-)-N-methyl-N-[7-(1-pyrrolidinyl)-1-oxaspiro[4.5] dec-8-yl]benzeneacetamide (U69,593), and conformational comparison with some kappa-agonists.Chem Pharm Bull (Tokyo). 1990;38:1815–1818.
Subramanian G, Patrelini MG, Portoghese PS, Ferguson DM. Molecular docking reveals a novel binding site model for fentanyl at the mu-opioid receptor.J Med Chem. 2000;43:381–391.
Mosberg HI, Fowler CB. Development and validation of opioid ligand-receptor interaction models: the structural basis of mu vs delta selectivity.J Pept Res. 2002;60:329–335.
Przydzial MJ, Pogozheva ID, Andrews SM, et al. Roles of residues 3 and 4 in cyclic tetrapeptide ligand recognition by the κ-opioid receptor.J Pept Res. 2005;65:333–342.
Mosberg HI, Omnaas JR, Medzihradsky F, Smith CB. Cyclic, disulfide- and dithioether-containing opioid tetrapeptides: development of a ligand with high delta opioid receptor selectivity and affinity.Life Sci. 1988;43:1013–1020.
Mosberg HI, Lomize AL, Wang C, et al. Development of a model for the δ-opioid receptor pharmacophore. 1. Conformationally restricted Tyr1 replacements in the cyclic δ receptor selective tetrapeptide Tyr-c[D-Cys-Phe-D-Pen]OH (JOM-13).J Med Chem 1994a;37:4371–4383.
Mosberg HI, Omnaas JR, Lomize A, et al. Development of a model for the δ-opioid receptor pharmacophore. 2. Conformationally restricted Phe3 replacements in the cyclic δ-receptor selective tetrapeptide Tyr-c[D-Cys-Phe-D-Pen]OH (JOM-13).J Med Chem. 1994b;37:4384–4391.
Mosberg HI, Dua RK, Pogozheva ID, Lomize AL. Development of a model for the δ-opioid receptor pharmacophore. 4. Residue 3 dehydrophenyl-alanine analogs of Tyr-c[D-Cys-Phe-D-Pen]OH (JOM-13) confirm required gauche orientation of aromatic sidechain.Biopolymers. 1996;39:287–296.
Lomize AL, Flippen-Anderson JL, George C, Mosberg HI. Conformational analysis of the δ receptor-selective, cyclic opioid peptide, Tyr-c[D-Cys-Phe-D-Pen]OH(JOM-13): comparison of X-ray crystallographic structures, molecular mechanics simulations and1H NMR data.J Am Chem Soc. 1994;116:429–436.
Ho JC.Development of a Model for the δ-opioid Receptor Pharmacophore [dissertation]. Ann Arbor, MI: University of Michigan; 1997.
McFadyen IJ, Ho JC, Mosberg HI, Traynor JR. Modifications of the cyclic mu receptor selective tetrapeptide Tyr-c[D-Cys-Phe-D-Pen]NH2 (Et): effects on opioid receptor binding and activation.J Pept Res. 2000;55:255–261.
Fowler CB III, Pogozheva ID III, Lomize AL III, LeVine H III, Mosberg HI. Complex of an active μ-opioid receptor with cyclic peptide agonist modeled from experimental constraints.Biochemistry. 2004a;43:15796–15810.
Mosberg HI. Complementarity of delta opioid ligand pharmacophore and receptor models.Biopolymers. 1999;51:426–439.
Law PY, Loh HH. Regulation of opioid receptor activities.J Pharmacol Exp Ther. 1999;289:607–624.
Chaturvedi K, Christoffers KH, Singh K, Howells RD. Structure and regulation of opioid receptors.Biopolymers. 2000;55:334–346.
Chavkin C, McLaughlin JP, Celver JP. Regulation of opioid receptor function by chronic agonist exposure: constitutive activity and desensitization.Mol Pharmacol. 2001;60:20–25.
Coward P, Wada HG, Falk MS, et al. Controlling signaling with a specifically designed Gi-coupled receptor.Proc Natl Acad Sci USA. 1998;95:352–357.
Pogozheva ID, Lomize AL, Mosberg HI. Opioid receptor 3-dimensional structures from distance geometry calculations with hydrogen bonding constraints.Biophys J 1998;75:612–634.
Surratt CK, Johnson PS, Moriwaki A, et al. Mu opiate receptor: charged transmembrane domain amino acids are critical for agonist recognition and intrinsic activity.J Biol Chem. 1994;269:20548–20553.
Befort K, Tabbara L, Bausch S, Chavkin C, Evans C, Kieffer BL. The conserved aspartate residue in the third putative transmembrane domain of the delta-opioid receptor is not the anionic counterpart for cationic opiate binding but is a constituent of the receptor binding site.Mol Pharmacol. 1996a;49:216–223.
Befort K, Tabbara L, Kling D, Maigret B, Kieffer BL. Role of aromatic transmembrane residues of the delta-opioid receptor in ligand recognition.J Biol Chem. 1996b;271:10161–10168.
Befort K, Zilliox C, Filliol D, Yue S, Kieffer BL. Constitutive activation of the delta opioid receptor by mutations in transmembrane domains III and VII.J Biol Chem 1999;274:18574–18581.
Bot G, Blake AD, Li S, Reisine T. Mutagenesis of the mouse delta opioid receptor converts(-)-buprenorphine from a partial agonist to an antagonist.J Pharmacol Exp Ther. 1998a;284:283–290.
Bot G, Blake AD, Li S, Reisine T. Mutagenesis of a single amino acid in the rat mu-opioid receptor discriminates ligand binding.J Neurochem. 1998b;70:358–365.
Spivak CE, Beglan CL, Seidleck BK, et al. Naloxone activation of mu-opioid receptors mutated at a histidine residue lining the opioid binding cavity.Mol Pharmacol. 1997;52:983–992.
Mansour A, Taylor LP, Fine JL, et al. Key residues defining the mu-opioid receptor binding pocket: a site-directed mutagenesis study.J Neurochem 1997;68:344–353.
Meng F, Ueda Y, Hoversten MT, et al. Creating a functional opioid alkaloid binding site in the orphanin FQ receptor through site-directed mutagenesis.Mol Pharmacol. 1998;53:772–777.
Li JG, Chen C, Yin J, et al. ASP147 in the third transmembrane helix of the rat mu opioid receptor forms ion-pairing with morphine and naltrexone.Life Sci. 1999;65:175–185.
Fukuda K, Terasako K, Kato S, Mori K. Identification of the amino acid residues involved in selective agonist binding in the first extracellular loop of the delta-and mu-opioid receptors.FEBS Lett. 1995;373:177–181.
Minami M, Onogi T, Nakagawa T, et al. DAMGO, a mu-opioid receptor selective ligand, distinguishes between mu-and kappa-opioid receptors at a different region from that for the distinction between mu-and delta-opioid receptors.FEBS Lett. 1995;364:23–27.
Wang JB, Johnson PS, Wu JM, Wang WF, Uhl GR. Human kappa opiate receptor second extracellular loop elevates dynorphin's affinity for human mu/kappa chimeras.J Biol Chem. 1994;269:2966–25969.
Xue JC, Chen C, Zhu J, et al. Differential binding domains of peptide and non-peptide ligands in the cloned rat kappa opioid receptor.J Biol Chem. 1994;269:30195–30199.
Ferguson DM, Kramer S, Metzger TG, Law PY, Portoghese PS. Isosteric replacement of acidic with neutral residues in extracellular loop-2 of the kappa-opioid receptor does not affect dynorphin A(1–13) affinity and function.J Med Chem. 2000;43:1251–1252.
Bonner G, Meng F, Akil H. Selectivity of mu-opioid receptor determined by interfacial residues near third extracellular loop.Eur J Pharmacol. 2000;403:37–44.
Xu H, Lu YF, Partilla JS, et al. Opioid peptide receptor studies. 11. Involvement of Tyr148, Trp318 and His319 of the rat mu-opioid receptor in binding of mu-selective ligands.Synapse. 1999;32:23–28.
Ulens C, Van Boven M, Daenens P, Tytgat J. Interaction of p-fluoro fentanyl on cloned human opioid receptors and exploration of the role of Trp-318 and His-319 in mu-opioid receptor selectivity.J Pharmacol Exp Ther. 2000;294:1024–1033.
Pepin MC, Yue SY, Roberts E, Wahlestedt C, Walker P. Novel “restoration of function” mutagenesis strategy to identify amino acids of the delta-opioid receptor involved in ligand binding.J Biol Chem. 1997;272:9260–9267.
Valiquette M, Vu HK, Yue SY, Wahlestedt C, Walker P. Involvement of Trp-284, Val-296, and Val-297 of the human delta-opioid receptor in binding of delta-selective ligands.J Biol Chem. 1996;271:18789–18796.
Hjorth SA, Thirstrup K, Grandy DK, Schwartz TW. Analysis of selective binding epitopes for the kappa-opioid receptor antagonist norbinaltorphime.Mol Pharmacol. 1995;47:1089–1094.
Cavalli A, Babey AM, Loh HH. Altered adenylyl cyclase responsiveness subsequent to point mutations of Asp 128 in the third transmembrane domain of the delta-opioid receptor.Neuroscience. 1999;93:1025–1031.
Li J, Huang P, Chen C, de Riel JK, Weinstein H, Liu-Chen LY. Constitutive activation of the mu opioid receptor by mutation of D3.49(164), but not D3.32(147): D3.49(164) is critical for stabilization of the inactive form of the receptor and for its expression.Biochemistry. 2001;40:12039–12050.
Mouledous L, Topham CM, Moisand C, Mollereau C, Meunier JC. Functional inactivation of the nociceptin receptor by alanine substitution of glutamine 286 at the C terminus of transmembrane segment VI: evidence from a site-directed mutagenesis study of the ORLI receptor transmembrane-binding domain.Mol Pharmacol. 2000;57:495–502.
DeCaillot FM, Befort K, Filliol D, Yue S, Walker P, Kieffer BL. Opioid receptor random mutagenesis reveals a mechanism for G protein-coupled receptor activation.Nat Struct Biol. 2003;10:629–636.
Spivak CE, Beglan CL, Zollner C, Surratt CK. Beta-Funaltrexamine, a gauge for the recognition site of wildtype and mutant H297Q mu-opioid receptors.Synapse. 2003;49:55–60.
Chen C, Yin J, Riel JK, et al. Determination of the amino acid residue involved in [3H]beta-funaltrexamine covalent binding in the cloned rat mu-opioid receptor.J Biol Chem. 1996;271:21422–21429.
Jones RM, Hjorth SA, Schwartz TW, Portoghese PS. Mutational evidence for a common kappa antagonist binding pocket in the wild-type kappa and mutant mu[K303E] opioid receptors.J Med Chem. 1998;41:4911–4914.
Larson DL, Jones RM, Hjorth SA, Schwartz TW, Portoghese PS. Binding of norbinaltorphimine (norBNI) congeners to wild-type and mutant mu and kappa opioid receptors: molecular recognition loci for the pharmacophore and address components of kappa antagonists.J Med Chem. 2000;43:1573–1576.
Fowler CB III, Pogozheva ID III, LeVine H III, Mosberg HI. Refinement of a homology model of the μ-opiodd receptor using distance constraints from intrinsic and engineered zinc-binding sites.Biochemistry. 2004b;43:8700–8710.
Metzger TG, Paterlini MG, Portoghese PS, Ferguson DM. An analysis of the conserved residues between halobacterial retinal proteins and G-protein coupled receptors: implications for GPCR modeling.J Chem Inf Comput Sci. 1996;36:857–861.
Strahs D, Weinstein H. Comparative modeling and molecular dynamics studies of the delta, kappa and mu opioid receptors.Protein Eng. 1997;10:1019–1038.
Alkorta I, Loew GH. A 3D model of the delta opioid receptor and ligand-receptor complexes.Protein Eng. 1996;9:573–583.
Subramanian G, Paterlini MG, Larson DL, Portoghese PS, Ferguson DM. Conformational analysis and automated receptor docking of selective arylacetamide-based kappa-opioid agonists.J Med Chem. 1998;41:4777–4789.
Paterlini G, Portoghese PS, Ferguson DM. Molecular simulation of dynorphin A-(1–10) binding to extracellular loop 2 of the kappa-opioid receptor: a model for receptor activation.J Med Chem. 1997;40:3254–3262.
Filizola M, Caakkonen L, Loew GH. 3D modeling, ligand binding and activations studies of the cloned mouse delta, mu, and kappa opioid receptors.Protein Eng. 1999a, 12:927–942.
Filizola M, Carteni-Farina M, Perez JJ. Molecular modeling study of the differential ligand-receptor interaction at the mu, delta and kappa opioid receptors.J Comput Aided Mol Des. 1999b;13:397–407.
Pogozheva ID, Lomize AL, Mosberg HI. The transmembrane 7 alpha-bundle of rhodopsin: distance geometry calculations with hydrogen bonding constraints.Biophys J. 1997;72:1963–1985.
Lomize AL, Pogozheva ID, Mosberg HI. Structural organization of G-protein-coupled receptors.J Comput Aided Mol Des. 1999;13:325–353.
Vaidehi N, Floriano WB, Trabanino R, et al. Prediction of structure and function of G protein-coupled receptors.Proc Natl Acad Sci USA. 2002;99:12622–12627.
Shacham S, Topf M, Avisar N, et al. Modeling the 3D structure of GPCRs from sequence.Med Res Rev. 2001;21:472–483.
Shi L, Javitch JA. The binding site of aminergic G protein-coupled receptors: the transmembrane segments and second extracellular loop.Annu Rev Pharmacol Toxicol. 2002;42:437–467.
Lawson Z, Wheatley M. The third extracellular loop of G-protein-coupled receptors: more than just a linker between 2 important transmembrane helices.Biochem Soc Trans. 2004;32:1048–1050.
Baker D, Sali A. Protein structure prediction and structural genomics.Science. 2001;294:93–96.
Palczewski K, Kumasaka T, Hori T, et al. Crystal structure of rhodopsin: a G protein-coupled receptor.Science. 2000;289:739–745.
Ballesteros JA, Shi L, Javitch JA. Structural mimicry in G protein-coupled receptors: implications of the high-resolution structure of rhodopsin for structure-function analysis of rhodopsin-like receptors.Mol Pharmacol. 2001;60:1–19.
Klabunde T, Hessler G. Drug design strategies for targeting G-protein-coupled receptors.ChemBioChem. 2002;3:928–944.
Bissantz C, Bernard P, Hibert M, Rognan D. Protein-based virtual screening of chemical databases. II. Are homology models of G-protein coupled receptors suitable targets?.Proteins. 2003;50:5–25.
Evers A, Klebe G. Ligand-supported homology modeling of g-protein-coupled receptor sites: models sufficient for successful virtual screening.Angew Chem Int Ed Engl. 2004a;43:248–251.
Horn F, Bettler E, Oliveira L, Campagne F, Cohen FE, Vriend G. GPCRDB information system for G protein-coupled receptors.Nucleic Acids Res. 2003;31:294–297.
Huang P, Li J, Chen C, Visiers I, Weinstein H, Liu-Chen LY. Functional role of a conserved motif in TM6 of the rat mu opioid receptor: constitutively active and inactive receptors result from substitutions of Thr6.34(279) with Lys and Asp.Biochemistry. 2001;40:13501–13509.
Sali A, Blundell TL. Comparative protein modeling by satisfaction of spatial restraints.J Mol Biol. 1993;234:779–815.
Fiser A, Sali A. Modeller: generation and refinement of homology-based protein structure models.Methods Enzymol. 2003;374:461–491.
Peitsch MC. ProMod and Swiss-Model: internet-based tools for automated comparative protein modelling.Biochem Soc Trans. 1996;24:274–279.
Lund O, Frimand K, Gorodkin J, et al. Protein distance constraints predicted by neural networks and probability density functions.Protein Eng. 1997;10:1241–1248.
Shindyalov IN, Bourne PE. Improving alignments in HM protocol with intermediate sequences. In: Forth Meeting on the Critical Assessment of Techniques for Protein Structure Prediction; 2000:A-92.
Lambert C, Leonard N, De Bolle X, Depiereux E. ESyPred3D: prediction of proteins 3D structures.Bioinformatics. 2002;18:1250–1256.
Kim DE, Chivian D, Baker D. Protein structure prediction and analysis using the Robetta server.Nucleic Acids Res. 2004;32:W526-W531.
Pieper U, Eswar N, Braberg H, et al. MODBASE, a database of annotated comparative protein structure models, and associated resources.Nucleic Acids Res. 2004;32:D217-D222.
Eswar N, John B, Mirkovic N, et al. Tools for comparative protein structure modeling and analysis.Nucleic Acids Res. 2003; 31:3375–3380.
John B, Sali A. Comparative protein structure modeling by iterative alignment, model building and model assessment.Nucleic Acids Res. 2003;31:3982–3992.
Riek RP, Rigoutsos I, Novotny J, Graham RM. Non-alpha-helical elements modulate polytopic membrane protein architecture.J Mol Biol. 2001;306:349–362.
Fiser A, Do RK, Sali A. Modeling of loops in protein structures.Protein Sci. 2000;9:1753–1773.
Chothia C, Lesk AM. Helix movements and the reconstruction of the haem pocket during the evolution of the cytochrome c family.J Mol Biol. 1985;182:151–158.
Meng EC, Bourne HR. Receptor activation: what does the rhodopsin structure tell us?Trends Pharmacol Sci. 2001;22:587–593.
Karnik SS, Gogonea C, Patil S, Saad Y, Takezako T. Activation of G-protein-coupled receptors: a common molecular mechanism.Trends Endocrinol Metab. 2003;14:431–437.
Ghanouni P, Steenhuis JJ, Farrens DL, Kobilka BK. Agonist-induced conformational changes in the G-protein-coupling domain of the beta 2 adrenergic receptor.Proc Natl Acad Sci USA. 2001a;98:5997–6002.
Farrens DL, Altenbach C, Yang K, Hubbell WL, Khorana HG. Requirement of rigid-body motion of transmembrane helices for light activation of rhodopsin.Science. 1996;274:768–770.
Gether U, Lin S, Ghanouni P, Ballesteros JA, Weinstein H, Kobilka BK. Agonists induce conformational changes in transmembrane domains III and VI of the beta2 adrenoceptor.EMBO J. 1997;16:6737–6747.
Dunham TD, Farrens DL. Conformational changes in rhodopsin: movement of helix F detected by site-specific chemical labeling and fluorescence spectroscopy.J Biol Chem. 1999;274:1683–1690.
Han M, Smith SO, Sakmar TP. Constitutive activation of opsin by mutation of methionine 257 on transmembrane helix 6.Biochemistry. 1998;37:8253–8261.
Cai K, Klein-Seetharaman J, Hwa J, Hubbell WL, Khorana HG. Structure and function in rhodopsin: effects of disulfide cross-links in the cytoplasmic face of rhodopsin on transducin activation and phosphorylation by rhodopsin kinase.Biochemistry. 1999;38:12893–12898.
Ghanouni P, Gryczynski Z, Steenhuis JJ, et al. Functionally different agonists induce distinct conformations in the G protein coupling domain of the beta 2 adrenergic receptor.J Biol Chem. 2001b;276:24433–24436.
Janz JM, Farrens DL. Rhodopsin activation exposes a key hydrophobic binding site for the transducin alpha-subunit C terminus.J Biol Chem. 2004;279:29767–29773.
Hubbell WL, Altenbach C, Hubbell CM, Khorana HG. Rhodopsin structure, dynamics, and activation: a perspective from crystallography, site-directed spin labeling, sulfhydryl reactivity, and disulfide crosslinking.Adv Protein Chem. 2003;63:243–290.
Lin SW, Sakmar TP. Specific tryptophan UV-absorbance changes are probes of the transition of rhodopsin to its active state.Biochemistry. 1996;35:11149–11159.
Altenbach C, Cai K, Khorana HG, Hubbell WL. Structural features and light-dependent changes in the sequence 306–322 extending from helix VII to the palmitoylation sites in rhodopsin: a sitedrected spin-labeling study.Biochemistry. 1999a;38:7931–7937.
Altenbach C, Klein-Seetharaman J, Hwa J, Khorana HG, Hubbell WL. Structural features and light-dependent changes in the sequence 59–75 connecting helices I and II in rhodopsin: a site-directed spinlabeling study.Biochemistry. 1999b;38:7945–7949.
Altenbach C, Cai K, Klein-Seetharaman J, Khorana HG, Hubbell WL. Structure and function in rhodopsin: mapping light-dependent changes in distance between residue 65 in helix TM1 and residues in the sequence 306–319 at the cytoplasmic end of helix TM7 and in helix H8.Biochemistry. 2001a;40:15483–15492.
Altenbach C, Klein-Seetharaman J, Cai K, Khorana HG, Hubbell WL. Structure and function in rhodopsin: mapping light-dependent changes in distance between residue 316 in helix 8 and residues in the sequence 60–75, covering the cytoplasmic end of helices TM1 and TM2 and their connection loop CL1.Biochemistry. 2001b;40:15493–15500.
Gether U, Kobilka BK. G protein-coupled receptors. II. Mechanism of agonist activation.J Biol Chem. 1998;273:17979–17982.
Devanathan S, Yao Z, Salamon Z, Kobilka B, Tollin G. Plasmon-waveguide resonance studies of ligand binding to the human beta 2-adrenergic receptor.Biochemistry. 2004;43:3280–3288.
Salamon Z, Cowell S, Varga E, Yamamura HI, Hruby VJ, Tollin G. Plasmon resonance studies of agonist/antagonist binding to the human delta-opioid receptor: new structural insights into receptor-ligand interactions.Biophys J. 2000;79:2463–2474.
Salamon Z, Hruby VJ, Tollin G, Cowell S. Binding of agonists, antagonists and inverse agonists to the human delta-opioid receptor produces distinctly different conformational states distinguishable by plasmon-waveguide resonance spectroscopy.J Pept Res. 2002;60:322–328.
Alves ID, Ciano KA, Boguslavski V, et al. Selectivity, cooperativity, and reciprocity in the interactions between the delta-opioid receptor, its ligands, and G-proteins.J Biol Chem. 2004a;279:44673–44682.
Alves ID, Cowell SM, Salamon Z, Devanathan S, Tollin G, Hruby VJ. Different structural states of the proteolipid membrane are produced by ligand binding to the human delta-opioid receptor as shown by plasmon-waveguide resonance spectroscopy.Mol Pharmacol. 2004b;65:1248–1257.
Tramontano A, Morea V. Exploiting evolutionary relationships for predicting protein structures.Biotechnol Bioeng. 2003;84:756–763.
Bates PA, Kelley LA, MacCallum RM, Sternberg MJ. Enhancement of protein modeling by human intervention in applying the automatic programs 3D-JIGSAW and 3D-PSSM.Proteins. 2001;39–46.
Li X, Jacobson MP, Friesner RA. High-resolution prediction of protein helix positions and orientations.Proteins. 2004;55:368–382.
Evers A, Gohlke H, Klebe G. Ligand-supported homology modelling of protein binding-sites using knowledge-based potentials.J Mol Biol. 2003;334:327–345.
Ward SD, Hamdan FF, Bloodworth LM, Wess J. Conformational changes that occur during M3 muscarinic acetylcholine receptor activation probed by the use of an in situ disulfide cross-linking strategy.J Biol Chem. 2002;277:2247–2257.
Swaminath G, Lee TW, Kobilka B. Identification of an allosteric binding site for Zn2+ on the beta2 adrenergic receptor.J Biol Chem. 2003;278:352–356.
Elling CE, Thirstrup K, Holst B, Schwartz TW. Conversion of agonist site to metal-ion chelator site in the beta(2)-adrenergic receptor.Proc Natl Acad Sci USA. 1999;96:12322–12327.
Holst B, Elling CE, Schwartz TW. Partial agonism through a zincion switch constructed between transmembrane domains III and VII in the tachykinin NK(1) receptor.Mol Pharmacol. 2000;58:263–270.
Lagerstrom MC, Klovins J, Fredriksson R, et al. High affinity agonistic metal ion binding sites within the melanocortin 4 receptor illustrate conformational change of transmembrane region 3.J Biol Chem. 2003;278:51521–51526.
Ewing TJ, Makino S, Skillman AG, Kuntz ID. DOCK 4.0: search strategies for automated molecular docking of flexible molecule databases.J Comput Aided Mol Des. 2001;15:411–428.
Jones G, Willett P, Glen RC, Leach AR, Taylor R. Development and validation of a genetic algorithm for flexible docking.J Mol Biol. 1997;267:727–748.
Rarey M, Wefing S, Lengauer T. Placement of medium-sized molecular fragments into active sites of proteins.J Comput Aided Mol Des. 1996;10:41–54.
Taylor RD, Jewsbury PJ, Essex JW. FDS: flexible ligand and receptor docking with a continuum solvent model and soft-core energy function.J Comput Chem. 2003;24:1637–1656.
Halgren TA, Murphy RB, Friesner RA, et al. Glide: a new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening.J Med Chem. 2004;47:1750–1759.
Venkatachalam CM, Jiang X, Oldfield T, Waldman M. LigandFit: a novel method for the shape-directed rapid docking of ligands to protein active sites.J Mol Graph Model. 2003;21:289–307.
Cavasotto CN, Abagan RA. Protein flexibility in ligand docking and virtual screening to protein kinases.J Mol Biol. 2004;337:209–225.
Perola E, Walters WP, Charifson PS. A detailed comparison of current docking and scoring methods on systems of pharmaceutical relevance.Proteins. 2004;56:235–249.
Halperin I, Ma B, Wolfson H, Nussinov R. Principles of docking: an overview of search algorithms and a guide to scoring functions.Proteins. 2004;47:409–443.
Kontoyianni M, McClellan LM, Sokol GS. Evaluation of docking performance: comparative data on docking algorithms.J Med Chem. 2004;47:558–565.
Carlson HA, McCammon JA. Accommodating protein flexibility in computational drug design.Mol Pharmacol. 2000;57:213–218.
Evers A, Klebe G. Successful virtual screening for a submicromolar antagonist of the neurokinin-1 receptor based on a ligand-supported homology model.J Med Chem. 2004b;47:5381–5392.
Heyl DL, Mosberg HI. Modification of the Phe3 aromatic moiety in delta receptor-selective dermorphin/deltorphin-related tetrapeptides: effects on opioid receptor binding.Int J Pept Protein Res. 1992;39:450–457.
Sebastian A, Bidlack JM, Jiang Q, et al. 14 beta-[(p-nitrocinnamoy 1)amino]morphinones, 14 beta-[(p-nitrocinnamoyl)amino]-7,8-dihydromorphinones, and their codeinone analogues: synthesis and receptor activity.J Med Chem. 1993;36:3154–3160.
Sagara T, Egashira H, Okamura M, Fujii I, Shimohigashi Y, Kanematsu K. Ligand recognition in mu opioid receptor: experimentally based modeling of mu opioid receptor binding sites and their testing by ligand docking.Bioorg Med Chem. 1996;4:2151–2166.
Chabre M, Breton J. Orientation of aromatic residues in rhodopsin: rotation of one tryptophan upon the meta I to meta II transition after illumination.Photochem Photobiol. 1979;30:295–299.
Baneres JL, Martin A, Hullot P, Girard JP, Rossi JC, Parello J Structure-based analysis of GPCR function: conformational adaptation of both agonist and receptor upon leukotriene B4 binding to recombinant BLT1.J Mol Biol. 2003;329:801–814.
Ruprecht JJ, Mielke T, Vogel R, Villa C, Schertler GF. Electron crystallography reveals the structure of metarhodopsin I.EMBO J. 2004;23:3609–3620.
Sammes PG, Taylor JB. Opioid receptors. In: Hansch C, ed.Comprehensive Medicinal Chemistry. Oxford, UK: Pergamon Press; 1990;805–844.
Schiller PW, Weltrowska G, Nguyen TM-D, Lemieux C, Chung NN, Lu Y. Conversion of μ-, δ- and κ-receptor selective opioid peptide agonists into μ-, δ-, and κ-receptor selective antagonists.Life Sci. 2003;73:691–698.
Huang P, Kim S, Loew G. Development of a common 3D pharmacophore for delta-opioid recognition from peptides and non-peptides using a novel computer program.J Comput Aided Mol Des. 1997;11:21–28.
Filizola M, Villar HO, Loew GH. Molecular determinants of non-specific recognition of delta, mu, and kappa opioid receptors.Bioorg Med Chem. 2001a;9:69–76.
Filizola M, Villar HO, Loew GH. Differentiation of delta, mu, and kappa opioid receptor agonists based on pharmacophore development and computed physicochemical properties.J Comput Aided Mol Des. 2001b;15:297–307.
Bernard D Jr, Coop A Jr, MacKerell AD Jr. 2D conformationally sampled pharmacophore: a ligand-based pharmacophore to differentiate delta opioid agonists from antagonists.J Am Chem Soc. 2003;125:3101–3107.
Author information
Authors and Affiliations
Additional information
Published: October 5, 2005
Rights and permissions
About this article
Cite this article
Pogozheva, I.D., Przydzial, M.J. & Mosberg, H.I. Homology modeling of opioid receptor-ligand complexes using experimental constraints. AAPS J 7, 43 (2005). https://doi.org/10.1208/aapsj070243
Received:
Accepted:
DOI: https://doi.org/10.1208/aapsj070243