Abstract
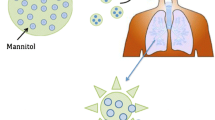
Non-cystic fibrosis bronchiectasis (NCFB) is a chronic respiratory disease associated with the high morbidity and mortality. Long-term intermittent therapy by inhalable antibiotics has recently emerged as an effective approach for NCFB treatment. However, the effective delivery of antibiotics to the lung requires administering a high dose to the site of infection. Herein, we investigated the novel inhalable silk-based microparticles as a promising approach to deliver high-payload ciprofloxacin (CIP) for NCFB therapy. Silk fibroin (SF) was applied to improve drug-payload and deposit efficiency of the dry powder particles. Mannitol was added as a mucokinetic agent. The dry powder inhaler (DPI) formulations of CIP microparticles were evaluated in vitro in terms of the aerodynamic performance, particle size distribution, drug loading, morphology, and their solid state. The optimal formulation (highest drug loading, 80%) exhibited superior aerosolization performance in terms of fine particle fraction (45.04 ± 0.84%), emitted dose (98.10 ± 1.27%), mass median aerodynamic diameter (3.75 ± 0.03 μm), and geometric standard deviation (1.66 ± 0.10). The improved drug loading was due to the electrostatic interactions between the SF and CIP by adsorption, and the superior aerosolization efficiency would be largely attributed to the fluffy and porous cotton-like property and low-density structure of SF. The presented results indicated the novel inhalable silk-based DPI microparticles of CIP could provide a promising strategy for the treatment of NCFB.








Similar content being viewed by others
References
Polverino E, Dimakou K, Hurst J, Angel Martinez-Garcia M, Miravitlles M, Paggiaro P, et al. The overlap between bronchiectasis and chronic airways diseases: state of the art and future directions. Eur Respir J. 2018;52:1800328. https://doi.org/10.1183/13993003.00328-2018.
Athanazio R, da Costa JC, de la Rosa Carrillo D, Martinez-Garcia MA. Current and future pharmacotherapy options for non-cystic fibrosis bronchiectasis. Expert Rev Respir Med. 2018;12:569–84.
Amaro R, Panagiotaraka M, Alcaraz V, Torres A. The efficacy of inhaled antibiotics in non-cystic fibrosis bronchiectasis. Expert Rev Respir Med. 2018;12:683–91.
Chalmers JD, Smith MP, McHugh BJ, Doherty C, Govan JR, Hill AT. Short- and long-term antibiotic treatment reduces airway and systemic inflammation in non-cystic fibrosis bronchiectasis. Am J Resp Crit Care. 2012;186:657–65.
Zhou Q, Leung SSY, Tang P, Parumasivam T, Loh ZH, Chan H-K. Inhaled formulations and pulmonary drug delivery systems for respiratory infections. Adv Drug Deliv Rev. 2015;85:83–99.
Ungaro F, d’Angelo I, Coletta C, d’Emmanuele di Villa Bianca R, Sorrentino R, Perfetto B, et al. Dry powders based on PLGA nanoparticles for pulmonary delivery of antibiotics: modulation of encapsulation efficiency, release rate and lung deposition pattern by hydrophilic polymers. J Control Release. 2012;157:149–59.
Quon BS, Goss CH, Ramsey BW. Inhaled antibiotics for lower airway infections. Ann Am Thorac Soc. 2014;11:425–34.
Barker AF, O’Donnell AE, Flume P, Thompson PJ, Ruzi JD, de Gracia J, et al. Aztreonam for inhalation solution in patients with non-cystic fibrosis bronchiectasis (AIR-BX1 and AIR-BX2): two randomised double-blind, placebo-controlled phase 3 trials. Lancet Respir Med. 2014;2:738–49.
Justo JA, Danziger LH, Gotfried MH. Efficacy of inhaled ciprofloxacin in the management of non-cystic fibrosis bronchiectasis. Ther Adv Respir Dis. 2013;7:272–87.
Cartlidge MK, Hill AT. Inhaled or nebulised ciprofloxacin for the maintenance treatment of bronchiectasis. Expert Opin Investig Drugs. 2017;26:1091–7.
De Soyza A, Aksamit T, Bandel T-J, Criollo M, Elborn JS, Operschall E, et al. RESPIRE 1: a phase III placebo-controlled randomised trial of ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis. Eur Respir J. 2018;51:1702052. https://doi.org/10.1183/13993003.02052-2017.
Aksamit T, De Soyza A, Bandel T-J, Criollo M, Elborn JS, Operschall E, et al. RESPIRE 2: a phase III placebo-controlled randomised trial of ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis. Eur Respir J. 2018;51:1702053. https://doi.org/10.1183/13993003.02053-2017.
Haworth C, Wanner A, Froehlich J, O’Neal T, Davis A, Gonda I, et al. Inhaled liposomal ciprofloxacin in patients with non-cystic fibrosis bronchiectasis and chronic Pseudomonas aeruginosa infection. J Aerosol Med Pulm Drug Deliv. 2017;30:29.
De Soyza A, Aksamit T. Ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis. Expert Opin Orphan Drugs. 2016;4:875–84.
Chalmers JD, Aliberti S, Blasi F. Management of bronchiectasis in adults. Eur Respir J. 2015;45:1446–62.
Polverino E, Goeminne PC, McDonnell MJ, Aliberti S, Marshall SE, Loebinger MR, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J. 2017;50:1–23.
Martínez-García MÁ, Máiz L, Olveira C, Girón RM, de la Rosa D, Blanco M, et al. Spanish guidelines on treatment of bronchiectasis in adults. Archivos de Bronconeumología (English Edition). 2018;54:88–98.
Claus S, Weiler C, Schiewe J, Friess W. How can we bring high drug doses to the lung? Eur J Pharm Biopharm. 2014;86:1–6.
Traini D, Young PM. Delivery of antibiotics to the respiratory tract: an update. Expert Opin Drug Deliv. 2009;6:897–905.
Lai SK, Wang Y-Y, Wirtz D, Hanes J. Micro- and macrorheology of mucus. Adv Drug Deliv Rev. 2009;61:86–100.
d’Angelo I, Conte C, La Rotonda MI, Miro A, Quaglia F, Ungaro F. Improving the efficacy of inhaled drugs in cystic fibrosis: challenges and emerging drug delivery strategies. Adv Drug Deliv Rev. 2014;75:92–111.
Wenk E, Merkle HP, Meinel L. Silk fibroin as a vehicle for drug delivery applications. J Control Release. 2011;150:128–41.
Mottaghitalab F, Farokhi M, Shokrgozar MA, Atyabi F, Hosseinkhani H. Silk fibroin nanoparticle as a novel drug delivery system. J Control Release. 2015;206:161–76.
Kim SY, Wong AH, Abou Neel EA, Chrzanowski W, Chan HK. The future perspectives of natural materials for pulmonary drug delivery and lung tissue engineering. Expert Opin Drug Deliv. 2015;12:869–87.
Schultz I, Vollmers F, Lühmann T, Rybak J-C, Wittmann R, Stank K, et al. Pulmonary insulin-like growth factor I delivery from trehalose and silk-fibroin microparticles. ACS Biomater Sci Eng. 2015;1:119–29.
Ong HX, Traini D, Salama R, Anderson SD, Daviskas E, Young PM. The effects of mannitol on the transport of ciprofloxacin across respiratory epithelia. Mol Pharm. 2013;10:2915–24.
Yang Y, Tsifansky MD, Shin S, Lin Q, Yeo Y. Mannitol-guided delivery of ciprofloxacin in artificial cystic fibrosis mucus model. Biotechnol Bioeng. 2011;108:1441–9.
Wilson R, Aksamit T, Aliberti S, De Soyza A, Elborn JS, Goeminne P, et al. Challenges in managing Pseudomonas aeruginosa in non-cystic fibrosis bronchiectasis. Respir Med. 2016;117:179–89.
Dhand R. The rationale and evidence for use of inhaled antibiotics to control Pseudomonas aeruginosa infection in non-cystic fibrosis bronchiectasis. J Aerosol Med Pulm Drug Deliv. 2018;31:121–38.
Hofmann S, Wong Po Foo CT, Rossetti F, Textor M, Vunjak-Novakovic G, Kaplan DL, et al. Silk fibroin as an organic polymer for controlled drug delivery. J Control Release. 2006;111:219–27.
Bayraktar O, Malay Ö, Özgarip Y, Batıgün A. Silk fibroin as a novel coating material for controlled release of theophylline. Eur J Pharm Biopharm. 2005;60:373–81.
Razuc M, Piña J, Ramírez-Rigo MV. Optimization of ciprofloxacin hydrochloride spray-dried microparticles for pulmonary delivery using design of experiments. AAPS PharmSciTech. 2018;19:3085–96. https://doi.org/10.1208/s12249-018-1137-6.
Weers J. Inhaled antimicrobial therapy—barriers to effective treatment. Adv Drug Deliv Rev. 2015;85:24–43.
Chew NYK, Chan HK. Use of solid corrugated particles to enhance powder aerosol performance. Pharm Res. 2001;18:1570–7.
Sahoo S, Chakraborti CK, Mishra SC, Nanda UN, Naik S. FTIR and XRD investigations of some fluoroquinolones. Int J Pharm Pharm Sci. 2011;3:165–70.
Bhardwaj A, Mehta S, Yadav S, Singh SK, Grobler A, Goyal AK, et al. Pulmonary delivery of antitubercular drugs using spray-dried lipid-polymer hybrid nanoparticles. Artif Cell Nanomed B. 2016;44:1544–55.
Wang Q, Dong ZF, Du YM, Kennedy JF. Controlled release of ciprofloxacin hydrochloride from chitosan/polyethylene glycol blend films. Carbohydr Polym. 2007;69:336–43.
Anderson JP. Morphology and crystal structure of a recombinant silk-like molecule, SLP4. Biopolymers. 1998;45:307–21.
Lammel AS, Hu X, Park SH, Kaplan DL, Scheibel TR. Controlling silk fibroin particle features for drug delivery. Biomaterials. 2010;31:4583–91.
Pritchard EM, Kaplan DL. Silk fibroin biomaterials for controlled release drug delivery. Expert Opin Drug Deliv. 2011;8:797–811.
Kaialy W, Hussain T, Alhalaweh A, Nokhodchi A. Towards a more desirable dry powder inhaler formulation: large spray-dried mannitol microspheres outperform small microspheres. Pharm Res. 2014;31:60–76.
Yucel T, Lovett ML, Keplan DL. Silk-based biomaterials for sustained drug delivery. J Control Release. 2014;190:381–97.
Kim EHJ, Chen XD, Pearce D. Surface composition of industrial spray-dried milk powders. 2. Effects of spray drying conditions on the surface composition. J Food Eng. 2009;94:169–81.
Meerdink G, Riet KVT. Modeling segregation of solute material during drying of liquid foods. AICHE J. 1995;41:732–6.
Chen XD, Sidhu H, Nelson M. Theoretical probing of the phenomenon of the formation of the outermost surface layer of a multi-component particle, and the surface chemical composition after the rapid removal of water in spray drying. Chem Eng Sci. 2011;66:6375–84.
Zhao ZY, Huang ZW, Zhang XJ, Huang Y, Cui YT, Ma C, et al. Low density, good flowability cyclodextrin-raffinose binary carrier for dry powder inhaler: anti-hygroscopicity and aerosolization performance enhancement. Expert Opin Drug Deliv. 2018;15:443–57.
Funding
This work was supported by National Natural Science Foundation of China (Grant No. 81503001), Guangdong Natural Science Foundation (Grant No. 2016A030313330), Guangdong Province Science and Technology Plan Project Public Welfare Fund and Ability Construction Project (Grant No. 2017A020215081, Grant No. 2016A020215069, Grant No. 2016A020215063), the Science and Technology Foundation of Guangzhou (Grant No.201707010103), and Yixian Scientific Research Project Set Sail (Grant No. YXQH201706).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 15 kb)
Rights and permissions
About this article
Cite this article
Liu, C., Lin, L., Huang, Z. et al. Novel Inhalable Ciprofloxacin Dry Powders for Bronchiectasis Therapy: Mannitol–Silk Fibroin Binary Microparticles with High-Payload and Improved Aerosolized Properties. AAPS PharmSciTech 20, 85 (2019). https://doi.org/10.1208/s12249-019-1291-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1291-5